Abstract
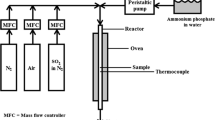
Pt-based catalysts cannot be used permanently for the diesel after-treatment system because the catalytic activity is decreased due to coarsening of Pt particles at high temperature of the exhaust gas. In this study, to prevent Pt-based catalyst from deactivation, Nd was added to the Pt/SiO2 catalyst, and the effect of the Nd addition on the catalytic activity was investigated. The Pt/SiO2 catalyst showed a high catalytic activity for the oxidation of NO but was severely deactivated after the fast thermal aging process. Pt crystallite size was increased and some Pt particles were buried in the SiO2 pore during the fast thermal aging process, which led to the decrease of catalytic activity. Nd-added Pt/SiO2 catalyst showed lower activity than Pt/SiO2 catalyst, but Pt–Nd/SiO2 catalyst maintained its catalytic activity after fast thermal aging process. It can be postulated that a stable Nd silicate, on which Pt particle is placed, protects SiO2 pores from destruction and so the number of the catalytically active sites remains nearly unchanged. As a result the Pt–Nd/SiO2 catalyst maintained its catalytic activity after fast thermal aging process.
Similar content being viewed by others
References
Landong, L., Lingling, Q., Jie, C., Jinjun, L., and Zhengping, H., Appl. Catal., B, 2009, vol. 88, p. 224.
Mergler, Y.J., Aalst, A., Delt, J., and Nieuwenhuys, B.E., J. Catal., 1996, vol. 161, p. 310.
Ronald, M.H. and Robert, J.F., Appl. Catal., A, 2001, vol. 221, p. 443.
Byung, C.C. and David, E.F., J. Mech. Sci. Technol., 2006, vol. 20, p. 1.
Jong, W.J., Byung, C.C., and Myung, T.L., J. Ind. Eng. Chem., 2008, vol. 14, p. 830.
Stanmore, B.R., Tschamber, V., and Brilhac, J.F., Fuel, 2008, vol. 87, p. 131.
Barry, A.A.L.S., Michiel, M., and Jacob, A.M., Catal. Rev., 2007, vol. 43, p. 489.
Junko, O., Akira, O., Shudong, W., Tetsuya, N., and Akihiko, O., Appl. Catal., B, 2003, vol. 43, p. 117.
Byung, C.C. and David, E.F., J. Ind. Eng. Chem., 2005, vol. 11, p. 1.
Byungchul, C., J. Ind. Eng. Chem., 2008, vol. 14, p. 499.
Qiang, W., So, Y.P., Linhai, D., and Jong, S.C., Appl. Catal., B, 2008, vol. 85, p. 10.
Zhiming, L. and Seong, I.W., Catal. Rev., 2006, vol. 48, p. 43.
Rui, M., Pierre, D., Patrick, D.C., Henry, M., Jean, T., and Gerald, D., J. Mol. Catal. A: Chem., 2004, vol. 221, p. 127.
Xue, E., Seshan, K., and Ommen, J.G., Appl. Catal., B, 1993, vol. 2, p. 183.
Xue, E., Seshan, K., and Ross, J.R.H., Appl. Catal., B, 1996, vol. 11, p. 65.
Song, T.O., Sang, M.K., Man, S.Y., Hyo, K.L., Gwon, K.Y., and Ho, I.L., React. Kinet. Catal. Lett., 2007, vol. 90, p. 339.
Michele, P., Marc, D., and Michel, S., Solid State Ionics, 1992, vol. 50, p. 31.
Calvin, H.B. and Wayne, L.S., J. Catal., 1983, vol. 81, p. 131.
Ozawa, Y., Tochihara, Y., Watanabe, A., Nagai, M., and Omi, S., Appl. Catal., A, 2004, vol. 258, p. 261.
Wang, F. and Lu, G., J. Power Sources, 2008, vol. 181, p. 120.
Leszek, K., Marek, W., and Marek, D., Mater. Chem. Phys., 2006, vol. 96, p. 353.
Duhan, S. and Aghamkar, P., Chin. Phys. Lett., 2009, vol. 26, p. 016106.
Tung, P. and Te, Y., Semicond. Sci. Technol., 2009, vol. 24, p. 095.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Kinetika i Kataliz, 2017, Vol. 58, No. 2, pp. 176–182.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Chung, YK., Lee, HI. Thermal deactivation mechanism and the effect of Nd addition on the deactivation of the Pt/SiO2 catalyst for NO oxidation reaction. Kinet Catal 58, 161–166 (2017). https://doi.org/10.1134/S0023158417020045
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158417020045