Abstract
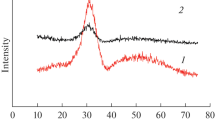
Thermal decomposition behavior was studied for Pr6O11, Pr2O3, Pr(OH3), and Pr2(CO3)3 · xH2O. In the course of transformation, the intermediate species were different for each starting material, as well as the surface area and morphology of the final thermal decomposition products. During thermal decomposition in the temperature range of 200°C up to 1400°C, mass losses for each species were attributed to the removal of hydroxide and carbonate species based on changes in the infrared bands for each powder for hydroxide groups at ≃3600 cm−1 and carbonate groups at ≃1300–1500 cm−1. X-ray diffraction analysis identified the final decomposition product at 1400°C for each species as Pr6O11 regardless of the starting material. A decrease in surface area and increase in equivalent particle radius for each final Pr6O11 product is attributed to sintering. The presence of hydroxyl and carbonate groups in the Pr6O11, Pr2O3, Pr(OH3), and Pr2 (CO3)3 · xH2O precursor dictate the morphology of thermally decomposed Pr6O11.
Similar content being viewed by others
References
Adachi, G., Imanaka, N., and Kang, Z.C., Binary Rare Earth Oxides, Dordrecht: Kluwer, 2004, pp. 1–55.
Gasgnier, M., Schiffmacher, G., Albert, L., and Caro, P.E., Preparation, Crystalline Properties and X-Ray Absorption Spectra of Rare Earth Oxides ROx (R = Ce, Pr, and Tb; 1.5 ≤ x ≤ 2), J. Less-Common Met., 1989, vol. 156, pp. 59–73.
Sharma, R., Hinode, H., and Eyring, L., A Study of the Decomposition of Praseodymium Hydroxy Carbonate and Praseodymium Carbonate Hydrate, J. Solid State Chem., 1991, vol. 92, no. 2, pp. 401–419.
Shears, E.C. and Ceram, L.I., A Study of Praseodymium Oxides by D.T.A. and Dilatometer, Br. Ceram. Trans. J., 1961, vol. 60, no. 11, pp. 773–782.
Caro, P.E., Sawyer, J.O., and Eyring, L., The Infrared Spectra of Rare Earth Carbonates, Spectrochim. Acta, Part A, 1972, vol. 28, no. 6, pp. 1167–1173.
Bernal, S., Botana, F.J., Garcia, R., and Rodriquez-Izquierdo, J.M., Behavior of Rare Earth Sesquioxides Exposed to Atmospheric Carbon Dioxide and Water, React. Solids, 1987, vol. 4, nos. 1–2, pp. 23–40.
Bernal, S., Diaz, J.A., Garcia, R., and Rodriguez-Izquierdo, J.M., Study of Some Aspects of the Reactivity of Lanthanum Oxide with Carbon Dioxide and Water, J. Mater. Sci., 1985, vol. 20, no. 2, pp. 537–541.
Bernal, S., Botana, F.J., Garcia, R., et al., Study of the Aging in Air of a Cubic Sample of Samaria, Mater. Res. Bull., 1987, vol. 22, no. 1, pp. 131–138.
Sastry, R.L.N., Yoganarasimhan, S.R., Mehrotra, P.N., and Rao, C.N.R., Preparation, Characterization, and Thermal Decomposition of Praseodymium, Terbium, and Neodymium Carbonates, J. Inorg. Nucl. Chem., 1966, vol. 28, no. 5, pp. 1165–1177.
Moscardini D’Assuncao, L., Ionashiro, M., Rasera, D.E., and Giolito, I., Thermal Decomposition of the Hydrated Basic Carbonates of Lanthanides and Yttrium in CO2 Atmosphere, Thermochim. Acta, 1993, vol. 219, nos. 1–2, pp. 225–233.
Haschke, J.M. and Eyring, L., Hydrothermal Equilibria and Crystal Growth of Rare Earth Oxides, Hydroxides, Hydroxynitrates, and Hydroxycarbonates, Inorg. Chem., 1971, vol. 10, no. 10, pp. 2267–2270.
Popa, M. and Kakihana, M., Praseodymium Oxide Formation by Thermal Decomposition of a Praseodymium Complex, Solid State Ionics, 2001, vols. 141–142, pp. 265–272.
Bernal, S., Botana, F.J., Garica, R., and Rodriguez-Izquierdo, J.M., Thermal Evolution of a Sample of Lanthanum Oxide Exposed to the Atmosphere, Thermochim. Acta, 1983, vol. 66, nos. 1–3, pp. 139–145.
Hussein, G.A.M., Formation of Praseodymium Oxide from the Thermal Decomposition of Hydrated Praseodymium Acetate and Oxalate, J. Anal. Appl. Pyrolysis, 1994, vol. 29, no. 1, pp. 89–102.
Swanson, H.E. and Fuyat, R.K., Standard X-Ray Diffraction Powder Patterns, Natl. Bur. Stand. Circ. (U. S.), 1953, vol. 2, p. 65.
Greis, O., Ziel, R., Breidenstein, B., et al., Crystal Structures of Cubic α-PrOF and Rhombohedral β-PrOF from X-Ray Powder Diffraction, Mater. Sci. Forum, 1994, vols. 166–169, no. 2, pp. 677–682.
Savin, V., Elyutin, A., Mikhailova, N., and Eremenko, Z., Thermochemical Study of the Process of Decomposition of Dysprosium, Lanthanum, and Neodymium Carbonates, Zh. Fiz. Khim., 1988, vol. 62, no. 5, pp. 1180–1187.
Sawyer, J., Caro, P., and Eyring, L., Hydroxycarbonates of the Lanthanide Elements, Rev. Chim. Miner., 1973, vol. 10, nos. 1–2, pp. 93–104.
Otsuka, K., Kunitomi, M., and Saito, T., Oxidation and Reduction of Praseodymium Oxides at Low Temperatures, Inorg. Chim. Acta, 1986, vol. 115, no. 1, pp. L31–L32.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Treu, B.L., Fahrenholtz, W.G. & O’Keefe, M.J. Thermal decomposition behavior of praseodymium oxides, hydroxides, and carbonates. Inorg Mater 47, 974–978 (2011). https://doi.org/10.1134/S0020168511090214
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168511090214