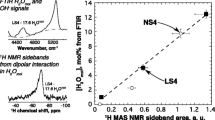
Abstract
Large-scale melting of the Earth’s early mantle under the effect of global impact processes was accompanied by the generation of volatiles, which concentration was mainly controlled by the interaction of main N, C, O, and H gas-forming elements with silicate and metallic melts at low oxygen fugacity (fO2), which predominated during metallic segregation and self-oxidation of magma ocean. The paper considers the application of Raman and IR (infrared) Fourier spectroscopy for revealing the mechanisms of simultaneous dissolution and relative contents of N, C, O, and H in glasses, which represent the quench products of reduced model FeO–Na2O–Al2O3–SiO2 melts after experiments at 4 GPa, 1550°C, and fO2 1.5–3 orders of magnitude below the oxygen fugacity of the iron—wustite buffer equilibrium (fO2(IW)). Such fO2 values correspond to those inferred for the origin and evolution of magma ocean. It was established that the silicate melt contains complexes with N–H bonds (NH3, NH +2 , NH -2 ), N2, H2, and CH4 molecules, as well as oxidized hydrogen species (OH– hydroxyl and molecular water H2O). Spectral characteristics of the glasses indicate significant influence of fO2 on the N–C–O–H proportion in the melt. They are expressed in a sharp decrease of NH +2 , NH -2 (O–NH2), OH–, H2O, and CH4 and simultaneous increase of NH -2 (≡Si–NH2) and NH3 with decreasing fO2. As a result, NH3 molecules become the dominant nitrogen compounds among N–C–H components in the melt at fO2 two orders of magnitude below fO2(IW), whereas molecular СН4 prevails at higher fO2. The noteworthy feature of the redox reactions in the melt is stability of the ОН– groups and molecular water, in spite of the sufficiently low fO2. Our study shows that the composition of reduced magmatic gases transferred to the planet surface has been significantly modified under conditions of self-oxidation of mantle and magma ocean.
Similar content being viewed by others
References
Y. E. Abe, T. Okuchi, K. Righter, and M. J. Drake, “Water in the early Earth,” in Origin of the Earth and Moon, Ed. by R. M. Canup and K. Righter (Univ. of Arizona Press, 2000), pp. 413–4335.
P. Ardia, M. M. Hirschmann, A. C. Withers, and B. D. Stanley, “Solubility of CH4 in a synthetic basaltic melt, with applications to atmosphere–magma ocean–core partitioning of volatiles and to the evolution of the Martian atmosphere,” Geochim. Cosmochim. Acta 114, 52–71 (2013).
D. Bouchard and Bale, C. W. “Simultaneous optimization of thermodynamical data for liquid iron alloys containing C, N, Ti, Si, Mn, Si, and P,” Metall. Trans. B, 26B, 467–483 (1995).
M. R. Carrol and Webster, J. D. “Solubilities of sulfur, noble gases, nitrogen, chlorine, and fluorine in magmas,” in Volatiles in Magmas, Ed. By M. R. Carroll and J. R. Holloway, (Min. Soc. Am., Washington D.C., 1994), Rev. Mineral. 30, 231–279 (1994).
P. F. Cartigny, F. Pineau, E. Petit, and M. Javoy, “Volatile (C, N, Ar) variability in MORB and the respective roles of mantle source heterogeneity and degassing: the case of the Southwest Indian Ridge,” Earth Planet. Sci. Lett. 194, 241–257 (2001).
A. Corgne, S. Keshav, B. J. Wood, W. F. McDonough, and Y. W. Fei, “Metal–silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion,” Geochim. Cosmochim. Acta 72, 574–589 (2008).
R. Dasgupta, H. Chi, N. Shimizu, A. S. Buono, and D. Walker, “Carbon solution and partitioning between metallic and silicate melts in a shallow magma ocean: implications for the origin and distribution of terrestrial carbon,” Geochim. Cosmochim. Acta 102, 191–212 (2013).
J. B. Dixon, E. M. Stolper, and J. R. Holloway, “An experimental study of water and carbon dioxide solubilities in mid–ocean ridge basaltic liquids. Part I: Calibration and solubility models,” J. Petrol. 36, 1607–1631 (1995).
D. J. Frost, U. Mann, Y. Asahara, and D. C. Rubie, “The redox state of the mantle during and just after core formation,” Phil. Trans. Royal Soc., A 366, 4315–4337 (2008).
E. M. Galimov, “Phenomenon of life: equilibrium and non–linearity,” Origin of Life and Evolution of the Biosphere 34 (6), 599–613 (2004).
E. M. Galimov, “Redox evolution of the earth caused by a multistage formation of its core,” Earth Planet. Sci. Lett. 233, 263–276 (2005).
E. M. Galimov, “Formation of the Moon and the Earth from a common supraplanetary gas–dust cloud (Lecture presented at the XIX All-Russia Symposium on Isotope Geochemistry on November 16, 2010),” Geochem. Int. 49 (6), 537–554 (2011).
M. M. Hirschmann, A. C. Withers, P. Ardia, and N. T. Foley, “Solubility of molecular hydrogen in silicate melts and consequences for volatile evolution of terrestrial planets,” Earth Planet Sci. Lett. 345–348, 38–48 (2012).
R. Hultgren, P. D. Desai, D. T. Hawkins, M. Gleiser, K. K. Kelley, Selected Values of the Thermodynamic Properties of Binary Alloys (American Society for Metals, Metals Park, Ohio, 1973).
M. Javoy, “The major volatile elements of the Earth: their origin, behavior, and fate,” Geophys. Res. Lett. 24, 177–180 (1997).
M. Javoy, E. Kaminski, F. Guyot, D. Andrault, C. Sanloup, M. Moreiraa, S. Labrosse, A. Jambon, P. Agrinier, A. Davaille, and C. Jaupart, “The chemical composition of the Earth: Enstatite chondrite models,” Earth Planet. Sci. Lett. 293, 259–268 (2010).
A. A. Kadik, F. Pineau, Y. A. Litvin, N. Jendrzejewski, I. Martinez, and M. Javoy, “Formation of carbon and hydrogen species in magmas at low oxygen fugacity during fluid–absent melting of carbon–bearing mantle,” J. Petrol. 45 (7), 1297–1310 (2004).
A. A. Kadik, N. A. Kurovskaya, Yu. A. Ignat’ev, N. N. Kononkova, V. V. Koltashev, and V. G. Plotnichenko, “Influence of oxygen fugacity on the solubility of carbon and hydrogen in FeO–Na2O–SiO2–Al2O3 melts in equilibrium with liquid iron at 1.5 GPa and 1400°C,” Geochem. Int. 48 (10), 953–960 (2010).
A. A. Kadik, N. A. Kurovskaya, Yu. A. Ignat’ev, N. N. Kononkova, V.V. Koltashev, and V.G. Plotnichenko, “Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO–Na2O–SiO2–Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400°C,” Geochem. Int. 49 (5), 429–438 (2011).
A. A. Kadik, Yu. A. Litvin, V. V. Koltashev, E. B. Kryukova, V. G. Plotnichenko, T. I. Tsekhonya, and N. N. Kononkova, “Solution behavior of reduced N?H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature,” Phys. Earth Planet. Inter. 214, 14–24 (2013).
A. A. Kadik, V. V. Koltashev, E. B. Kryukova, V. G. Plotnichenko, T. I. Tsekhonya, and N. N. Kononkova, “Solution behavior of C–O–H volatiles in FeO–Na2O–Al2O3–SiO2 melts in equilibrium with liquid iron alloy and graphite at 4 GPa and 1550°C,” Geochem. Int. 52 (9), 707–725 (2014).
A. A. Kadik, V. V. Koltashev, E. B. Kryukova, V. G. Plotnichenko, T. I. Tsekhonya, and N. N. Kononkova, “Solubility of nitrogen, carbon, and hydrogen in FeO–Na2O–Al2O3–SiO2 melt and liquid iron alloy: influence of oxygen fugacity,” Geochem. Int. 53 (11), 867–887 (2015).
S. C. Kohn, R. A. Brooker, and R. Dupree, “13C MAS NMR: a method for studying CO2 speciation in glasses,” Geochim. Cosmochim. Acta 55, 3879–3884 (1991).
Yu. A. Litvin, “Distribution of pressure up to 40 kbar and temperature up to 1500°C in the solid–state cell of large useful volume,” Pribory Tekhnika Experimenta, No. 5, 207–209 (1979).
Yu. A. Litvin, Physico–Chemical Study of Melting Relations of the Deep–Seated Earth’s Substance (Nauka, Moscow, 1991) (in Russian).
R. Machida and Y. Abe, “Terrestrial planet formation through accretion of sublimatic icy planetesimals in cold nebula,” Astrophys. J. 716, 1252–1262 (2010).
B. Marty “The origins and concentrations of water, carbon, nitrogen, and noble gases on Earth,” Earth Planet. Sci. Lett. 313–314, 56–66 (2012).
M. Mercier, A. Di Muro, N. Metrich, D. Giordano, O. Belhadj, and Ch. W. Mandeville, “Spectroscopic analysis (FTIR, Raman) of water in mafic and intermediate glasses and glass inclusions,” Geochim. Cosmochim. Acta 74, 5641–5656 (2010).
A. Morbidelli, J. Chambers, J. I. Lunine, J. M. Petit, F. Robert, G. B. Valsecchi, and K. E. Cyr, “Source regions and timescales for the delivery of water to Earth,” Meteorit. Planet. Sci. 35, 1309–1320 (2000).
K. Muralidharan, P. Deymier, M. Stimpfl, N. H. de Leeuw, and M. J. Drake, “Origin of water in the inner Solar System: a kinetic Monte Carlo study of water adsorotion on forsterite,” Icarus 198, 400–407 (2008).
B. O. Mysen, “Silicate–COH melt and fluid structure, their physicochemical properties, and partitioning of nominally refractory oxides between melts and fluids,” Lithos 148, 228–246 (2012).
B. O. Mysen, “Structure–property relationships of COHN–saturated silicate melt coexisting with COHN fluid: a review of in–situ, high–temperature, high–pressure experiments,” Chem. Geol. 346, 113–124 (2013).
B. O. Mysen and Fogel, M. L. “Nitrogen and hydrogen isotope compositions and solubility in silicate melts in equilibrium with reduced (N + H)–bearing fluids at high pressure and temperature: effects of melt structure,” Am. Mineral. 95, 987–999 (2010).
B. O. Mysen and P. Richet, Silicate Glasses and Melts: Properties and Structure, Dev. Geochem. 10 (2005).
B. O. Mysen and Virgo, D. “Volatiles in silicate melts at high pressure and temperature: 2. Water in melts along the join NaAlO2–SiO2 and a comparison of solubility mechanisms of water and fluorine,” Chem. Geol. 57, 333–358 (1986).
B. O. Mysen, M. L. Fogel, G. D. Cody, and P. L. Morrill, “Solution behavior of reduced C-O-H volatiles in silicate melts at high pressure and temperature,” Geochim. Cosmochim. Acta 73, 1696–1710 (2009).
B. O. Mysen, K. Kumamoto, G. D. Cody, and M. L. Fogel, “Solubility and solution mechanisms of C-O-H volatiles in silicate melt with variable redox conditions and melt composition at upper mantle temperatures and pressures,” Geochim. Cosmochim. Acta 75, 6183–6199 (2011).
S. Newman, E. M. Stolper, and S. Epstein, “Measurement of water in rhyolitic glasses: Calibration of an infrared spectroscopic technique,” Am. Mineral. 71, 1527–1541 (1986).
A. Ricolleau, Y. Fei, A. Corgne, J. Siebert, and J. James Badro, “Oxygen and silicon contents of Earth’s core from high pressure metal–silicate partitioning experiments,” Earth Planet Sci. Lett. 310, 409–421 (2011).
M. Roskosz, M. A. Bouhifd, A. P. Jephcoat, B. Marty, and B. O. Mysen, “Nitrogen solubility in molten metal and silicate at high pressure and temperature,” Geochim. Cosmochim. Acta 121, 15–28 (2013).
B. D. Stanley, M. M. Hirschmann, and A. C. Withers, “Solubility of C–O–H volatiles in graphite–saturated Martian basalts,” Geochim. Cosmochim. Acta 129, 54–76 (2014).
E. Stolper, “Water in silicate glasses: an infrared spectroscopic study,” Contrib. Mineral. Petrol. 81, 1–17 (1982).
D. T. Wetzel, S. D. Jacobsen, M. J. Rutherford, E. H. Hauri, and A. E. Saal, “Degassing of reduced carbon from planetary basalts,” Proc. Natl. Acad. Sci. (2013). http://dx.doi.org/ doi 10.1073/ pnas.1219266110
B. J. Wood, “Carbon in the core,” Earth Planet. Sci. Lett. 117, 593–607 (1993).
B. J. Wood, M. J. Walter, and J. Wade, “Accretion of the Earth and segregation of its core,” Nature 441, 825–833 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadik, A.A., Koltashev, V.V., Kryukova, E.B. et al. Application of IR and Raman spectroscopy for the determination of the role of oxygen fugacity in the formation of N–С–О–Н molecules and complexes in the iron-bearing silicate melts at high pressures. Geochem. Int. 54, 1175–1186 (2016). https://doi.org/10.1134/S0016702916130073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702916130073