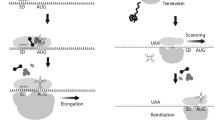
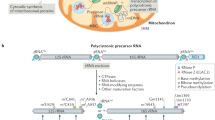
Abstract
Mitochondria are essential organelles of eukaryotic cell that provide its respiratory function by means of the electron transfer chain. Expression of mitochondrial genes is organized in a bacterial-like manner; however multiple evolutionary differences are observed between the two systems, including translation initiation machinery. This review is dedicated to the mitochondrial translation initiation factor 3 (IF3mt), which plays a key role in the protein synthesis in mitochondria. Involvement of IF3mt in human health and disease is discussed.
Similar content being viewed by others
Abbreviations
- CTD:
-
C-terminal domain
- CTE:
-
C-terminal extension
- fMet:
-
formylmethionine
- IF3mt:
-
mitochondrial translation initiation factor 3
- LSU:
-
large subunit
- NC:
-
normal chow
- NTD:
-
N-terminal domain
- NTE:
-
N-terminal extension
- PA:
-
physically active
- PD:
-
Parkinson’s disease
- SED:
-
sedentary
- SSU:
-
small subunit
- TACO1:
-
translational activator of cytochrome c oxidase 1
- TUFM:
-
thermo-unstable elongation factor, mitochondrial
- WD:
-
Western diet
References
Calvo, S. E., and Mootha, V. K. (2010) The mitochondrial pro- teome and human disease, Annu. Rev. Genomics Hum. Genet., 11, 25–44, doi:https://doi.org/10.1146/annurev-genom-082509-141720.
Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., Schreier, P. H., Smith, A. J., Staden, R., and Young, I. G. (1981) Sequence and organization of the human mitochondrial genome, Nature, 290, 457–465, doi:https://doi.org/10.1038/290457a0.
Christian, B. E., and Spremulli, L. L. (2012) Mechanism of protein biosynthesis in mammalian mitochondria, Biochim. Biophys. Acta, 1819, 1035–1054, doi:https://doi.org/10.1016/j.bbagrm.2011.11.009.
Amunts, A., Brown, A., Toots, J., Scheres, S. H., and Ramakrishnan, V. (2015) The structure of the human mitochondrial ribosome, Science, 348, 95–98, doi:https://doi.org/10.1126/science.aaa1193.
Montoya, J., Ojala, D., and Attardi, G. (1981) Distinctive features of the 5’-terminal sequences of the human mitochondrial mRNAs, Nature, 290, 465–470, doi:https://doi.org/10.1038/290465a0.
Salinas-Giege, T., Giege, R., and Giege, P. (2015) tRNA biology in mitochondria, Int. J. Mol. Sci., 16, 4518–4559, doi: https://doi.org/10.3390/ijms16034518.
Kuzmenko, A., Atkinson, G. C., Levitskii, S., Zenkin, N., Tenson, T., Hauryliuk, V., and Kamenski, P. (2014) Mitochondrial translation initiation machinery: conservation and diversification, Biochimie, 100, 132–140, doi: https://doi.org/10.1016/j.biochi.2013.07.024.
Biou, V., Shu, F., and Ramakrishnan, V. (1995) X-ray crys- tallography shows that translational initiation factor IF3 consists of two compact alpha/beta domains linked by an alpha-helix, EMBO J., 14, 4056–4064.
Petrelli, D., LaTeana, A., Garofalo, C., Spurio, R., Pon, C. L., and Gualerzi, C. O. (2001) Translation initiation factor IF3: two domains, five functions, one mechanism? EMBO J., 20, 4560–4569, doi:https://doi.org/10.1093/emboj/20.16.4560.
Ayyub, S. A., Dobriyal, D., and Varshney, U. (2017) Contributions of the N- and C-terminal domains of initiation factor 3 to its functions in the fidelity of initiation and antiassociation of the ribosomal subunits, J. Bacteriol., 199, 1–12, doi:https://doi.org/10.1128/JB.00051-17.
Koc, E. C., and Spremulli, L. L. (2002) Identification of mammalian mitochondrial translational initiation factor 3 and examination of its role in initiation complex formation with natural mRNAs, J. Biol. Chem., 277, 35541–35549, doi: https://doi.org/10.1074/jbc.M202498200.
Haque, M. E., and Spremulli, L. L. (2008) Roles of the N- and C-terminal domains of mammalian mitochondrial initiation factor 3 in protein biosynthesis, J. Mol. Biol., 384, 929–940, doi:https://doi.org/10.1016/j.jmb.2008.09.077.
Brown, A., Amunts, A., Bai, X. C., Sugimoto, Y., Edwards, P. C., Murshudov, G., Scheres, S. H., and Ramakrishnan, V. (2014) Structure of the large ribosomal subunit from human mitochondria, Science, 346, 718–722, doi: https://doi.org/10.1126/science.1258026.
Bhargava, K., and Spremulli, L. L. (2005) Role of the N- and C-terminal extensions on the activity of mammalian mitochondrial translational initiation factor 3, Nucleic Acids Res., 33, 7011–7018, doi:https://doi.org/10.1093/nar/gki1007.
Haque, M. E., Grasso, D., and Spremulli, L. L. (2008) The interaction of mammalian mitochondrial translational initiation factor 3 with ribosomes: evolution of terminal extensions in IF3mt, Nucleic Acids Res., 36, 589–597, doi: https://doi.org/10.1093/nar/gkm1072.
Yu, N. J., and Spremulli, L. L. (1998) Regulation of the activity of chloroplast translational initiation factor 3 by NH2- and COOH-terminal extensions, J. Biol. Chem., 273, 3871–3877, doi:https://doi.org/10.1074/jbc.273.7.3871.
Derbikova, K., Kuzmenko, A., Levitskii, S., Klimontova, M., Chicherin, I., Baleva, M. V., Krasheninnikov, I. A., and Kamenski, P. (2018) Biological and evolutionary significance of terminal extensions of mitochondrial translation initiation factor 3, Int. J. Mol. Sci., 19, 1–15, doi: https://doi.org/10.3390/ijms19123861.
Christian, B. E., and Spremulli, L. L. (2009) Evidence for an active role of IF3mt in the initiation of translation in mammalian mitochondria, Biochemistry, 48, 3269–3278, doi: https://doi.org/10.1021/bi8023493.
Hussain, T., Llacer, J. L., Wimberly, B. T., Kieft, J. S., and Ramakrishnan, V. (2016) Large-scale movements of IF3 and tRNA during bacterial translation initiation, Cell, 167, 133–144, doi:https://doi.org/10.1016/j.cell.2016.08.074.
Haque, M. E., Koc, H., Cimen, H., Koc, E. C., and Spremulli, L. L. (2011) Contacts between mammalian mitochondrial translational initiation factor 3 and ribosomal proteins in the small subunit, Biochim. Biophys. Acta, 1814, 1779–1784, doi:https://doi.org/10.1016/j.bbapap.2011.09.013.
Koripella, R. K., Sharma, M. R., Haque, M. E., Risteff, P., Spremulli, L. L., and Agrawal, R. K. (2019) Structure of human mitochondrial translation initiation factor 3 bound to the small ribosomal subunit, Science, 12, 76–86, doi: https://doi.org/10.1016/j.isci.2018.12.030.
Lopez-Alonso, J. P., Fabbretti, A., Kaminishi, T., Iturrioz, I., Brandi, L., Gil-Carton, D., Gualerzi, C. O., Fucini, P., and Connell, S. R. (2017) Structure of a 30S pre-initiation complex stalled by GE81112 reveals structural parallels in bacterial and eukaryotic protein synthesis initiation pathways, Nucleic Acids Res., 45, 2179–2187, doi:https://doi.org/10.1093/nar/gkw1251.
Lee, D. E., Brown, J. L., Rosa, M. E., Brown, L. A., Perry, R. A., Washington, T. A., and Greene, N. P. (2016) Translational machinery of mitochondrial mRNA is promoted by physical activity in Western diet-induced obese mice, Acta Physiol. (Oxf.), 218, 167–177, doi:https://doi.org/10.1111/apha.12687.
Yokokawa, T., Kido, K., Suga, T., Isaka, T., Hayashi, T., and Fujita, S. (2018) Exercise-induced mitochondrial biogenesis coincides with the expression of mitochondrial translation factors in murine skeletal muscle, Physiol. Rep., 6, 1–13, doi:https://doi.org/10.14814/phy2.13893.
Gaweda-Walerych, K., and Zekanowski, C. (2013) The impact of mitochondrial DNA and nuclear genes related to mitochondrial functioning on the risk of Parkinson’s dis- ease, Curr. Genomics, 14, 543–559, doi:https://doi.org/10.2174/1389202914666131210211033.
Abahuni, N., Gispert, S., Bauer, P., Riess, O., Kruger, R., Becker, T., and Auburger, G. (2007) Mitochondrial translation initiation factor 3 gene polymorphism associated with Parkinson’s disease, Neurosci. Lett., 414, 126–129, doi: https://doi.org/10.1016/j.neulet.2006.12.053.
Behrouz, B., Vilarino-Guell, C., Heckman, M. G., Soto-Ortolaza, A. I., Aasly, J. O., Sando, S., Lynch, T., Craig, D., Uitti, R. J., Wszolek, Z. K., Ross, O. A., and Farrer, M. J. (2010) Mitochondrial translation initiation factor 3 polymorphism and Parkinson’s disease, Neurosci. Lett., 486, 228–230, doi:https://doi.org/10.1016/j.neulet.2010.09.059.
Anvret, A., Ran, C., Westerlund, M., Thelander, A. C., Sydow, O., Lind, C., Hakansson, A., Nissbrandt, H., Galter, D., and Belin, A. C. (2010) Possible involvement of a mitochondrial translation initiation factor 3 variant causing decreased mRNA levels in Parkinson’s disease, Parkinson’s Dis., 2010, 1–5, doi:https://doi.org/10.4061/2010/491751.
Kuzmenko, A., Derbikova, K., Salvatori, R., Tankov, S., Atkinson, G. C., Tenson, T., Ott, M., Kamenski, P., and Hauryliuk, V. (2016) Aim-less translation: loss of Saccharomyces cerevisiae mitochondrial translation initiation factor mIF3/Aim23 leads to unbalanced protein syn- thesis, Sci. Rep., 6, 1–9, doi:https://doi.org/10.1038/srep18749.
Funding
The work was funded by the Russian Science Foundation (project 18-74-00016); the work of E.B.D. was financially supported by the State Basic Research Program no. 0108-2019-004 for the Institute of Developmental Biology, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflict of interest.
Compliance with ethical standards. This article does not contain description of studies involving animals or human participants performed by any of the authors.
Additional information
Published in Russian in Biokhimiya, 2019, Vol. 84, No. 10, pp. 1401-1409
Rights and permissions
About this article
Cite this article
Chicherin, I.V., Baleva, M.V., Levitskii, S.A. et al. Mitochondrial Translation Initiation Factor 3: Structure, Functions, Interactions, and Implication in Human Health and Disease. Biochemistry Moscow 84, 1143–1150 (2019). https://doi.org/10.1134/S0006297919100031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297919100031