Abstract
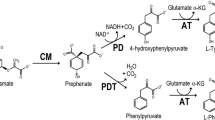
K m for L-phenylalanine, L-glutamic acid, L-aspartic acid, and the corresponding keto acids were calculated, as well as V max was measured for the following pairs of substrates: L-phenylalanine-2-ketoglutarate, L-phenylalanine-oxaloacetate, L-glutamic acid-phenylpyruvate, and L-aspartic acid-phenylpyruvate for aminotransferases PAT1, PAT2, and PAT3 from Erwinia carotovora catalyzing transamination of phenylpyruvate. The ping-pong bi-bi mechanism was shown for the studied aminotransferases. The substrate inhibition (K s) of PAT3 with 2-ketoglutarate and oxaloacetate was 10.23 ± 3.20 and 3.73 ± 1.99 mM, respectively.
It was shown that L-β-(N-benzylamino)alanine was a competitive inhibitor with respect to L-phenylalanine for PAT1 (K i = 0.32 ± 0.07 mM, K m = 0.45 ± 0.1 mM, V max = 11. 6 ± 0.4 U/mg) at 25 mM concentration of 2-ketoglutarate in the reaction medium. L-β-(N-methylamino)alanine is a noncompetitive inhibitor with respect to L-phenylalanine for PAT3 (K I = 138.4 ± 95.4 mM, K m = 13.7 ±3.9 mM, V max = 18.6 ± 4.1 U/mg) at 2 mM concentration of 2-ketoglutarate in the reaction medium. L-stereo isomers of nonprotein analogues of aromatic amino acids were studied as substrates for PAT1, PAT2, and PAT3. L-β-(2-Br-phenyl)alanine, L-β-(4-Br-phenyl)alanine, L-β-(2-F-phenyl)alanine, and L-(2-F)tryptophan were good substrates for all three aminotransferases; L-α-methyl-β-(2-Br-phenyl)alanine and L-O-benzyltyrosine were substrates only for PAT3; L-β-(4-F-phenyl)alanine was a substrate for PAT1 and PAT3. Thus, these analogues of aromatic amino acids can be stereoselectively synthesized using the studied aminotransferases in the presence of the corresponding keto acids.
Similar content being viewed by others
References
Braunshtein, A.E. and Kritsman, M.G., Enzimologiya, 1937, vol. 2, pp. 129–137.
Collier, R.H. and Kohlhaw, G., J. Bacteriol., 1972, vol. 112, no. 1, pp. 365–371.
Mavrides, C. and Orr, W., J. Biol. Chem., 1975, vol. 250, no. 11, pp. 4128–4133.
Gelfand, D.H. and Steinberg, R.A., J. Bacteriol., 1977, vol. 130, pp. 429–440.
Powell, J.T. and Morrison, J.F., J. Biochem., 1978, vol. 87, pp. 391–400.
Weigent, D.A. and Nester, E.W., J. Biochem., 1976, vol. 251, no. 22, pp. 6974–6980.
Nester, E.W. and Montoya, A.L., J. Bacteriol., 1976, vol. 126, no. 2, pp. 699–705.
Sung, M., Tanizawa, K., Tanaka, H., Kuramitsu, S., Kagamiyama, H., and Soda, K., J. Bacteriol., 1990, vol. 172, no. 3, pp. 1345–1351.
Fazel, A.M. and Jensen, R.A., J. Bacteriol., 1979, vol. 140, no. 2, pp. 580–587.
Lee, C. and Desmazeaud, M.J., J. Gen. Microbiol., 1985, vol. 131, pp. 459–467.
Whitaker, R.J., Gaines, C.G., and Jensen, R.A., J. Biol. Chem., 1982, vol. 257, no. 22, pp. 13550–13556.
Ziehr, H. and Kula, M-R., J. Biotechnol., 1985, vol. 3, nos. 1–2, pp. 19–31.
Paris, C.G. and Magasanik, B., J. Bacteriol., 1981, vol. 145, no. 1, pp. 266–271.
Abou-Zeid, A., Euverink, G.J.W., Hessels, G.I., Jensen, R.A., and Dijkhuizen, L., Appl. Environ. Microbiol., 1995, vol. 61, no. 4, pp. 1298–1302.
Rijnen, L., Bonneau, S., and Yvon, M., Appl. Environ. Microbiol., 1999, vol. 65, no. 11, pp. 4873–4880.
Xing, R.Y. and Whitman, W.B., J. Bacteriol., 1992, vol. 174, no. 2, pp. 541–548.
Andreotti, G., Cubellis, M.V., Nitti, G., Sannia, G., Mai, X., Marino, G., and Adams, M.W.W., Eur. J. Biochem., 1994, vol. 220, pp. 543–549.
Matsui, I., Matsui, E., Sakai, Y., Kikuchi, H., Kawarabayasi, Y., Urai, H., Kawaguchii, S., Kuramitsui, S., and Harata, K., J. Biol. Chem., 2000, vol. 275, no. 7, pp. 4871–4879.
Ward, D.E., De Vos, W.M., and Van Der Oost, J., J. Archaea, 2002, vol. 1, pp. 133–141.
Cardenas-Fernandez, M., Lopez, C., Alvaro, G., and Lopez-Santin, J., J. Biochem. Engin., 2012, vol. 63, pp. 15–21.
US Patent No. 4783403, 1988.
US Patent No. 4745059, 1988.
AM Patent No. 2479A, 2010.
Paloyan, A.M., Hambardzumyan, A.A., and Halebyan, Gh.P., Biochemistry (Moscow), 2012, vol. 77, no. 1, pp. 98–104.
Peterson, G., Methods Enzymol., 1983, vol. 91, no. 1, pp. 95–119.
Cornish-Bowden, A., Fundamentals of Enzyme Kinetics, London: Portland, 1977.
King, E.L. and Altman, C., J. Phys. Chem., 1956, vol. 60, pp. 1375–1378.
Kirchner, J., in Tonkosloinaya khromatografiya (Thin Layer Chromatography), Moscow: Mir, 1981.
Velick, S.F. and Vavra, J., J. Biol. Chem., 1962, vol. 237, no. 7, pp. 2109–2122.
Ambartsumyan, A.A. and Bezirdzhyan, Kh.O., Biochemistry (Moscow), 1994, vol. 59, no. 9, pp. 1027–1032.
Montemartini, J.A. and Santome, J.J., Cazzulot and C. Nowicki, J. Biochem., 1993, vol. 292, pp. 901–906.
Oganesyan, A.M., Biol. Zh. Armen., 2009, vol. 64, no. 4, pp. 101–104.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.M. Paloyan, L.A. Stepanyan, S.A. Dadayan, A.A. Hambardzumyan, Gh.P. Halebyan, A.S. Saghiyan, 2013, published in Prikladnaya Biokhimiya i Mikrobiologiya, 2013, Vol. 49, No. 2, pp. 129–135.
Rights and permissions
About this article
Cite this article
Paloyan, A.M., Stepanyan, L.A., Dadayan, S.A. et al. Catalytic properties of enzymes from Erwinia carotovora involved in transamination of phenylpyruvate. Appl Biochem Microbiol 49, 106–112 (2013). https://doi.org/10.1134/S0003683813020129
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683813020129