Abstract
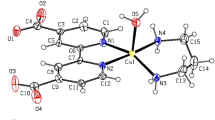
Copper chelates with tridentate ligands containing pyridine or pyrazole ring at the azomethine or azo fragment were synthesized by chemical electrochemical methods, and their structure was characterized by the EXAFS spectra. Thermal magnetochemical analysis of the complexes revealed antiferromagnetic exchange interaction in all complexes. The exchange interaction parameter of the complex containing an N-tosylamino group in the ortho position with respect to the azomethine group is much lesser than that of the corresponding complex having an oxygen atom in the same position. The copper chelate derived from azopyrazole ligand shows low-temperature ferromagnetic phase transition.
Similar content being viewed by others
References
Minkin, V.I., Olekhnovich, L.P., and Zhdanov, Yu.A., Molekulyarnyi dizain tautomernykh sistem (Molecular Design of Tautomeric Systems), Rostov-on-Don: Rostov. Gos. Univ., 1977.
Minkin, V.I., Olekhnovich, L.P., and Zhdanov, Yu.A., Molecular Design of Tautomeric Compounds, Dordrecht: Kluwer Academic, 1988.
Minkin, V.I., Olekhnovich, L.P., and Zhdanov, Yu.A., Acc. Chem. Res., 1981, vol. 14, no. 7, p. 210.
Garnovskii, A.D. and Vasil’chenko, I.S., Usp. Khim., 2002, vol. 71, no. 11, p. 1064.
Garnovskii, A.D. and Vasil’chenko, I.S., Usp. Khim., 2005, vol. 74, no. 3, p. 211.
Ovcharenko, V.I. and Sagdeev, R.Z., Usp. Khim., 1999, vol. 68, no. 5, p. 381.
Kahn, O., Molecular Magnetism, New York: Wiley, 1993.
Gatteschi, D., Sessoli, R., and Cornia, A., Comprehensive Coordination Chemistry II, McCleverty, J.A. and Meyer, T.J., Eds., Amsterdam: Elsevier, 2003, vol. 2, p. 393.
Single-Molecule Magnets and Related Phenomena, (Structure and Bonding series, vol. 122), Winpenny, R., Ed., Berlin: Springer, 2006.
Proc. 10th Int. Conf. on Molecule-Based Magnets (ICMM 2006), Polyhedron, 2007, vol. 26, nos. 9–11.
Uraev, A.I., Vasilchenko, I.S., Ikorskii, V.N., Shestakova, T.A., Burlov, A.S., Lyssenko, K.A., Vlasenko, V.G., Kuz’menko, T.A., Divaeva, L.N., Pirog, I.V., Borodkin, G.S., Uflyand, I.E., Antipin, M.Yu., Ovcharenko, V.I., Garnovskii, A.D., and Minkin, V.I., Mendeleev Commun., 2005, vol. 15, no. 4, p. 133.
Garnovskii, A.D., Burlov, A.S., Lukov, V.V., Zaletov, V.G., Asmaev, O.T., Levchenkov, S.I., Amarskii, E.G., and Garnovskii, D.A., Koord. Khim., 1996, vol. 22, no. 11, p. 838.
Burlov, A.S., Koshchienko, Yu.V., Ikorskii, V.N., Vlasenko, V.G., Zarubin, I.A., Uraev, A.I., Vasil’chenko, I.S., Garnovskii, D.A., Borodkin, G.S., Nikolaevskii, S.A., and Garnovskii, A.D., Zh. Neorg. Khim., 2006, vol. 51, no. 7, p. 1143.
Burlov, A.S., Ikorskii, V.N., Uraev, A.I., Koshchienko, Yu.V., Vasil’chenko, I.S., Garnovskii, D.A., Borodkin, G.S., Nikolaevskii, S.A., and Garnovskii, A.D., Russ. J. Gen. Chem., 2006, vol. 76, no. 8, p. 1282.
Garnovskii, A.D., Ikorskii, V.N., Uraev, A.I., Vasilchenko, I.S., Burlov, A.S., Garnovskii, D.A., Lyssenko, K.A., Vlasenko, V.G., Shestakova, T.E., Koshchienko, Yu.V., Kuz’menko, T.A., Divaeva, L.N., Bubnov, M.P., Rybalkin, V.P., Korshunov, O.Yu., Pirog, I.V., Borodkin, G.S., Bren, V.A., Uflyand, I.E., Antipin, M.Yu., and Minkin, V.I., J. Coord. Chem., 2007, vol. 60, no. 14, p. 1493.
Garnovskii, A.D., Uraev, A.I., and Minkin, V.I., Arkivoc, 2004, part 3, p. 29.
Shkol’nikova, L.M. and Porai-Koshits, M.A., Itogi Nauki Tekh., 1982, vol. 16, p. 117.
Skopenko, V.V., Amirkhanov, V.M., Sliva, T.Yu., Vasil’chenko, I.S., Anpilova, E.L., and Garnovskii, A.D., Usp. Khim., 2004, vol. 73, no. 8, p. 797.
Garnovskii, A.D., Alekseenko, V.A., Kogan, V.A., Bolotin, B.M., Osipov, O.A., Yusman, T.A., and Chernova, N.I., Koord. Khim., 1977, vol. 3, no. 4, p. 500.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Willey, 1997, 5th ed., part B.
Tahir, M.N., Ülkü, D., Atakol, O., and Akay, A., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1996, vol. 52, no. 11, p. 2676.
Mathur, R.P., Pradhan, M.V., and Mathur, P., Oriental J. Chem., 1993, vol. 9, no. 4, p. 320.
Kochubei, D.I., Babanov, Yu.A., Zamaraev, K.I., Vedrinskii, R.V., Kraizman, V.L., Kulipanov, G.N., Mazalov, L.N., Skrinskii, A.N., Fedorov, V.K., Khel’mer, B.Yu., and Shuvaev, A.T., Rentgenospektral’nyi metod izucheniya struktury amorfnykh tel: EXAFS-spektroskopiya (X-Ray Absorption Method of Studying the Structure of Amorphous Sciences: EXAFS Spectroscopy), Novosibirsk: Nauka, Sib. Otd., 1988.
Newville, M., J. Synchrotron Rad., 2001, vol. 8, part 2, p. 96.
Zabinsky, S.I., Rehr, J.J., Ankudinov, A., Albers, R.C., and Eller, M.Y., Phys. Rev. B, 1995, vol. 52, no. 4, p. 2995.
Allen, F.H., Acta Crystallogr., Sect. B, 2002, vol. 58, no. 3, p. 380.
Hernandez-Molina, R. and Menderos, A., Comprehensive Coordination Chemistry II, McCleverty, J.A. and Meyer, T.J., Eds., Amsterdam: Elsevier, 2003, vol. 1, p. 411.
Emelius, L.C., Cupertino, D.C., Harris, S.G., Owens, S., Parsons, S., Swart, R.M., Tasker, P.A., and White, D.J., J. Chem. Soc., Dalton Trans, 2001, no. 8, p. 1239.
Garnovskii, A.D., Vasil’chenko, I.S., and Garnovskii, D.A., Sovremennye aspekty sinteza metallokompleksov. Osnovnye ligandy i metody (Modern Aspects of the Synthesis of Metal Complexes. Ligands and Methods), Rostov-on-Don: LaPO, 2000.
Synthetic Coordination and Organometallic Chemistry, Garnovskii, A.D. and Kharisov, B.I., Eds., New York: Marcel Dekker, 2003.
Chernova, N.I., Ryabokobylko, Yu.S., Brudz’, V.G., and Bolotin, B.M., Zh. Org. Khim., 1971, vol. 7, no. 8, p. 1680.
Garnovskii, D.A., Antsyshkina, A.S., Sadikov, G.G., Sousa, A., Burlov, A.S., Vasil’chenko, I.S., and Garnovskii, A.D., Zh. Neorg. Khim., 1998, vol. 43, no. 11, p. 1852.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Burlov, A.I. Uraev, V.N. Ikorskii, S.A. Nikolaevskii, Yu.V. Koshchienko, I.S. Vasil’chenko, D.A. Garnovskii, V.G. Vlasenko, Ya.V. Zubavichus, L.N. Divaeva, G.S. Borodkin, A.D. Garnovskii, 2008, published in Zhurnal Obshchei Khimii, 2008, Vol. 78, No. 6, pp. 1002–1007.
Rights and permissions
About this article
Cite this article
Burlov, A.S., Uraev, A.I., Ikorskii, V.N. et al. Molecular design of new magnetically active copper complexes with heteroaromatic schiff bases and azo compounds. Russ J Gen Chem 78, 1230–1235 (2008). https://doi.org/10.1134/S1070363208060224
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363208060224