Abstract
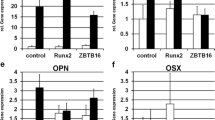
Runx2/core binding factor alpha 1 (Cbfa1) and Osterix (Osx) are osteoblast-specific transcription factors essential for the development of a mature osteoblast phenotype and are thought to activate osteoblast marker genes in vivo to produce a bone-specific matrix. Dexamethasone (Dex) is known to be a potent stimulator of osteoblastic differentiation in vitro, however, the exact role is still unclear. To investigate the mechanisms of the stimulation of osteoblastic differentiation by Dex, we evaluated the effects of Dex on proliferation and mineralization as well as on mRNA expression of Cbfa1, Osx and osteoblast marker genes, osteocalcin (OC) and bone sialoprotein (BSP) mRNAs in differentiating foetal rat calvarial cells (FRCC), which were cultured for 35 days in the presence or absence of 10−7 M Dex. Treatment of FRCC with Dex resulted in the stimulation of cell proliferation and increased the number of cells, which are able to produce bone-like nodules with a mineralized matrix when compared to untreated controls. Northern blot analysis revealed that, in the absence of Dex, Cbfa1 mRNA expressed at day 8, while Osx mRNA expressed at day 15. Subsequently expression of these mRNAs increased up to day 21, followed by constant expression during the culture period. The expression of OC and BSP mRNAs appeared to be synchronous with that of Osx mRNA and was detectable at day 15 with an increase thereafter. The presence of Dex resulted in an induction in Cbfa1 and Osx mRNA expression. The former appeared at day 5 and the latter appeared at day 11. Subsequently expression of Cbfa1 and Osx mRNAs increased up to day 15 with a decrease thereafter. Expression of OC and BSP mRNAs appeared to be coincident with that of Osx mRNA and was detectable at day 11 and reached a maximum at day 15 followed by constant expression. These observations indicate that induction of Cbfa1 and Osx mRNAs by Dex may be followed by activation of osteoblast marker genes such as OC and BSP mRNAs to produce a bone-specific matrix that subsequently becomes mineralized. Thus, it is likely that Dex may promote osteoblastic differentiation and mineralization of FRCC by inducing the expression of Cbfa1 and Osx genes in vitro.
Similar content being viewed by others
References
Csordas G, Thomas AP, Hajnoczky G (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18: 96–108.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC (1999) Bid-induced confor-mational change of Bax is response for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144: 891–901.
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42.
Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC (1998) Bax-induced cytochrome c release from mitochondria is independent of the permeability transition pore but highly dependent on Mg 2 +ions. J Cell Biol 143: 217–224.
Eskes R, Desagher S, Antonsson B, Martinou JC (2000) Bid induces oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20: 929–935.
Gajkowska B, Wojewodzka U (2002) A novel immunoelectron embedment free electron microscopy reveals association of apoptosis-regulating proteins with subcellular structures. Histochem J 34: 441–446.
Gajkowska B, Puvion E, Bernhard W(1977) Unusual perinucleolar accu-mulation of ribonucleoprotein granules induced by camptothecin in isolated liver cells. J Ultrastructure Res 60: 335–347.
Godlewski MM, Gajkowska B, Lamparska-Przybysz M, Motyl T (2002) Colocalization of BAX with BID and VDAC-1 in nimesulide-induced apoptosis of human colon adenocarcinoma COLO 205 cells. Anti-Cancer Drugs 13: 1–14.
Godlewski MM, Motyl MA,Gajkowska B, Motyl T (2001) Subcellular redistribution of BAX during apoptosis induced by anticancer drugs. Anti-Cancer Drugs 12: 607–617.
Green DR(2000) Apoptotic pathways: Paper wraps stone blunts scissors. Cell 102: 1–4.
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309–1312.
He Q, Lee DI, Rong R, Yu M, Luo X, Klein M, El-Deiry WS, Huang Y, Hussain A, Sheikh MS (2002) Endoplasmic reticulum calcium pool depletion-induced apoptosis in coupled with activation of the death receptor 5 pathway. Oncogene 21: 2623–2633.
Horwitz SB (1974) Novel inhibitors of RNA synthesis. Fed Proc 33: 2281–2287.
Horwitz SB, Chang CK, Grollman AP (1971) Studies on camptothecin. I. Effects of nucleic acid and protein synthesis. Mol Pharmocol 7: 632–644.
Jiang S, Chow SC, Nicotera P, Orrenius S (1994) Intracellular Ca 2 +sig-nals activate apoptosis in thymocytes: Studies using the Ca (2 +)-ATPase inhibitor thapsigargin. Exp Cell Res 212: 84–92.
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: Coordination of gene transcriptional and translational controls. Genes Dev 13: 1211–1233.
Kluck R, Degli Esposti M, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR, Newmeyer DD (1999) The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J Cell Biol 147: 809–822.
Kolek O, Gajkowska B, Godlewski MM, Motyl T (2003) Colocalization of apoptosis regulating proteins in mouse mammary epithelial HC11 cells exposed to TGF-â 1. Eur J Cell Biol 82: 303–312.
Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18: 44–52.
Kumar A, Wu RS (1973) Role of ribosomal RNA transcription in ribosome processing in HeLa cells. J Mol Biol 80: 265–276.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86: 147–157.
Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G (1998) Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281: 2027–2031.
Nakamura K, Bossy-Wetzel E, Burns K, Marc PF, Lozyk M, Goping IS, Opas M, Bleackey C, Green, DR, Michalak M (2000) Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol 150: 731–740.
Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG (2002) Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca 2 +stores. J Biol Chem 277: 9219–9225.
Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: The unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13: 349–356.
Pink JJ, Wuerzberger-Davis S, Tagliarino C, Planchon SM, Yang X, Froelich CJ, Boothman DA (2000) Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during beta-lapachone-mediated apoptosis. Exp Cell Res 255: 144–155.
Recher L, Chan H, Briggs L, Parry N (1972) Ultrastructural changes inducible with the plant alkaloid camptothecin. Cancer Res 32: 2495–2501.
Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca 2 +close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747.
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca 2 +responses. Science 280: 1763–1766.
Scarrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) Bax and Bak regulation of endoplasmic reticulum Ca 2 +: A control point for apoptosis. Science 300: 135–139.
Shay JW, Werbin H (1992) New evidence for the insertion of mitochon-drial DNA into the human genome: Significance for cancer and aging. Mutat Res 275: 227–235.
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G (1999a) Mitochondrial release of caspase-2 and-9 during the apoptotic process. J Exp Med 189: 381–394.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999b) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446.
Susin S, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G (1996) Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 184: 1331–1341.
Szalai G, Krishnamurthy R, Hajnoczky G (1999) Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J 18: 6349–6361.
Vander Heiden MG, Thompson CB (1999) Bcl-2 proteins: Regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol 1: E209–E215.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102: 43–53.
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 14: 2060–2071.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic Bax and Bak: A requisite gateway to mitochon-drial dysfunction and death. Science 292: 727–730.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 275: 1129–1132.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Igarashi, M., Kamiya, N., Hasegawa, M. et al. Inductive Effects of Dexamethasone on the Gene Expression of Cbfa1, Osterix and Bone Matrix Proteins During Differentiation of Cultured Primary Rat Osteoblasts. Histochem J 35, 3–10 (2004). https://doi.org/10.1023/B:HIJO.0000020883.33256.fe
Issue Date:
DOI: https://doi.org/10.1023/B:HIJO.0000020883.33256.fe