Abstract
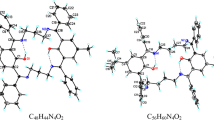
Condensation of 4-tert-butyl-2,6-diformylphenol with 1,2-diaminobenzene in ethanol is accompanied by partial reduction of the azomethine double bonds to form symmetrical macrocyclic Schiff"s base containing the alternating >C=N and >CH—NH fragments. In solution, this compound exists as the only isomer in which two endocyclic hydrogen atoms are bound to the oxygen atoms of the phenol groups and two other endocyclic H atoms are attached to the nitrogen atoms of the CH2—NH fragments. All endocyclic protons are involved in hydrogen bonding and undergo rapid exchange with each other at room temperature. In the crystal, the planar macrocyclic molecules are arranged in closely packed stacks. The steric hindrances resulting from overlapping of the bulky tert-butyl groups are eliminated through rotation of the molecules with respect to each other in the adjacent layers. Study of the potential energy surface for the Schiff"s base under consideration by the DFT method demonstrated that the structure corresponding to the global minimum is similar to that found in solution. However, the isolated molecule is nonplanar, its macrocycle adopting a ladder conformation. The local minimum on the potential energy surface whose energy is 2.6 kcal mol–1 higher than that of the global minimum corresponds to the zwitterionic structure in which all four endocyclic hydrogen atoms are attached to the nitrogen atoms and the macrocycle adopts a tub conformation. Flattening of the ring is considered as a consequence of stacking interactions between the molecules in the crystal.
Similar content being viewed by others
References
A. Yu. Chernyadyev, Yu. A. Ustynyuk, O. V. Yazev, E. A. Kataev, M. D. Reshetova, A. A. Sidorov, G. G. Aleksandrov, V. N. Ikorskii, V. M. Novotortsev, S. E. Nefedov, I. L. Eremenko, and I. I. Moiseev, Izv. Akad. Nauk, Ser. Khim., 2001, 2334 [Russ. Chem. Bull., Int. Ed., 2001, 50, 2445].
J.-M. Lehn, Supramolecular Chemistry. Concepts and Perspectives, Weinheim, New York-Basel-Cambridge; VCH Venlagsgesellschaft mbH, Tokyo, 1995.
R. H. Holm, P. Kennepohl, and E. I. Solomon, Chem. Rev., 1996, 96, 2239.
L. F. Lindoy, Pure Appl. Chem., 1989, 61, 1575.
Molecular Electronics, Ed. G. J. Fishwell, Wiley, New York, 1992.
A. Jasat and D. Dolphin, Chem. Rev., 1997, 97, 2267.
H. Okawa, H. Furutachi, and D. E. Fenton, Coord. Chem. Rev., 1998, 174, 51.
K. Brychcy, K. Draeger, K.-J. Jens, M. Tilset, and U. Behrens, Chem. Ber., 1994, 127, 1817.
Y. Tian, J. Tong, G. Frenzen, and J.-Y. Sun, J. Org. Chem., 1999, 64, 1442.
A. Aguiari, E. Bullita, U. Castellato, P. Guerriero, S. Tamburini, and P. A. Vigato, Inorg. Chim. Acta, 1992, 202, 157.
D. Neuhaus, J. Magn. Res., 1983, 53, 109.
M. Kinns and J. K. M. Sanders, J. Magn. Res., 1984, 56, 518.
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpin, and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, 1.
A. J. Atkins, A. J. Blake, A. Marin-Becerra, S. Parsons, I. Ruiz-Ramirez, and M. Schroeder, Chem. Commun., 1996, 457.
L. F. Lindoy, G. V. Meehan, and N. Svenstrup, Synthesis, 1998, 1029.
G. M. Sheldrick, SADABS. Program for Scaling and Correction of Area Detector Data, University of Göttingen, Göttingen (Germany), 1997.
G. M. Sheldrick, SHELXS97. Program for the Solution of Crystal Structures, University of Göttingen, Göttingen (Germany), 1997.
G. M. Sheldrick, SHELXL97. Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen (Germany), 1997.
SMART (Control) and SAINT (Integration) Software, Ver. 5.0, Bruker AXS Inc., Madison, WI, 1997.
D. N. Laikov, Ph. D. (Phys.-Mat.) Thesis, M. V. Lomonosov Moscow State University, Moscow, 2000 (in Russian)
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151.
M. S. Nechaev, I. V. Borisova, N. N. Zemlyanskii, D. N. Laikov, and Yu. A. Ustynyuk, Izv. Akad. Nauk, Ser. Khim., 2000, 1850 [Russ. Chem. Bull., Int. Ed., 2000, 49, 1823].
L. Yu. Ustynyuk, Yu. A. Ustynyuk, D. N. Laikov, and V. V. Lunin, Izv. Akad. Nauk, Ser. Khim., 1999, 2248 [Russ. Chem. Bull., 1999, 48, 2222 (Engl. Transl.)].
Yu. A. Ustynyuk, L. Yu. Ustynyuk, D. N. Laikov, and V. V. Lunin, J. Organomet. Chem., 2000, 597, 182.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ustynyuk, Y.A., Borisova, N.E., Nosova, V.M. et al. Binuclear and polynuclear transition metal complexes with macrocyclic ligands. 2. New macrocyclic Schiff"s base in the reaction of 4-tert-butyl-2,6-diformylphenol with 1,2-diaminobenzene. Synthesis and structural, spectroscopic, and theoretical study. Russ Chem Bull 51, 488–498 (2002). https://doi.org/10.1023/A:1015564403703
Issue Date:
DOI: https://doi.org/10.1023/A:1015564403703