Abstract
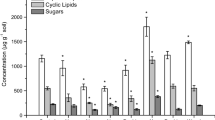
The influence of site fertility on soil microbial biomass and activity is not well understood but is likely to be complex because of interactions with plant responses to nutrient availability. We examined the effects of long-term (8 yr) fertilization and litter removal on forest floor microbial biomass and N and C transformations to test the hypothesis that higher soil resource availability stimulates microbial activity. Microbial biomass and respiration decreased by 20–30 % in response to fertilization. Microbial C averaged 3.8 mg C/g soil in fertilized, 5.8 mg C/g in control, and 5.5 mg C/g in litter removal plots. Microbial respiration was 200 µg CO2-C g−1 d−1 in fertilized plots, compared to 270 µg CO2-C g−1 d−1 in controls. Gross N mineralization and N immobilization did not differ among treatments, despite higher litter nutrient concentrations in fertilized plots and the removal of substantial quantities of C and N in litter removal plots. Net N mineralization was significantly reduced by fertilization. Gross nitrification and NO3 − immobilization both were increased by fertilization. Nitrate thus became a more important part of microbial N cycling in fertilized plots even though NH4 + availability was not stimulated by fertilization.
Soil microorganisms did not mineralize more C or N in response to fertilization and higher litter quality; instead, results suggest a difference in the physiological status of microbial biomass in fertilized plots that influenced N transformations. Respiration quotients (qCO2, respiration per unit biomass) were higher in fertilized plots (56 µg CO2-C mg C−1 d−1) than control (48 µg CO2-C mg C−1 d −1) or litter removal (45 µg CO2-C mg C−1 d−1), corresponding to higher microbial growth efficiency, higher proportions of gross mineralization immobilized, and lower net N mineralization in fertilized plots. While microbial biomass is an important labile nutrient pool, patterns of microbial growth and turnover were distinct from this pool and were more important to microbial function in nitrogen cycling.
Similar content being viewed by others
References
Aber JD, Melillo JM Nadelhoffer KJ, Pastor J & Boone RD (1991) Factors controlling nitrogen cycling and nitrogen saturation in northern temperate forest ecosystems. Ecol. Appl. 1: 303-315
Aber JD, McDowell W, Nadelhoffer K, Magill A, Bernston G, Kamakea M, McNulty S, Currie W, Rustad L & Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. Bioscience 48: 921-933
Aerts RC, Bakker C & DeCaluwe H (1992) Root turnover as determinant of the cycling of C, N, and P in a dry heathland ecosystem. Biogeochemistry 15: 175-190
Anderson T-H (1994) Physiological analysis of microbial communities in soil: Applications and limitations. In: Ritz K, Dighton J & Giller KE (Eds) Beyond the Biomass: Compositional and Functional Analysis of Soil Microbial Communities (pp 67-76). John Wiley and Sons, New York
Anderson T-H & Domsch KH (1985) Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol. Fert. Soils 1: 81-89
Arnebrandt K, Bååth E, Söderström B & Nohrstedt HO (1996) Soil microbial activity in eleven Swedish coniferous forests in relation to site fertility and nitrogen fertilization. Scand. Journ. For. Res. 11: 1-6
Bloom AF, Chapin FS III & MOnney HA (1985) Resource limitation in plants-an economic analogy. Ann. Rev. Ecol. Systematics 16: 363-392
Bohlen PJ, Groffman PM, Driscoll CT, Fahey TJ & Siccama TG (2001) Plant-soil-microbial interactions in a northern hardwood forest. Ecology, in press
Bottner P, Sallih Z & Billes G (1988) Root activity and carbon metabolism in soils. Biol. Fert. Soils 7: 71-78
Brookes PC, Landman A, Pruden G & Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method for measuring microbial biomass nitrogen in soil. Soil Biol. Biochem. 17: 837-842
Brooks PD, Stark JM, McInteer BB & Preston T (1989) A diffusion method to prepare soil KCl extracts for 15N analysis. Soil Sci. Soc. Am. Journ. 53: 1707-1711
Burke IC, Reiners WA & Schimel DS (1989) Organic matter turnover in a sagebrush steppe landscape. Biogeochemistry 7: 11-31
Chapin FS III (1980) The mineral nutrition of wild plants. Ann. Rev. Ecol. Systematics 11: 233-260
Chapin FS III (1991) Integrated responses of plants to stress. Bioscience 41: 29-36
Clein JS & Schimel JP (1995) Nitrogen turnover and availability during succession from alder to poplar in Alaskan taiga forests. Soil Biol. Biochem. 27: 743-752
D'Elia CF, Steudler PA & Corwin N (1977) Determination of total nitrogen in aqueous samples using persulfate digestion. Limnol. Oceanogr. 22: 760-764
Davidson EA, Hart SC, Shanks CA & Firestone MK (1991) Measuring gross nitrogen mineralization, immobilization, and nitrification by 15N isotopic pool dilution in intact soil cores. Journ. Soi. Sci. 42: 335-349
Davidson EA, Hart SC & Firestone MK (1992) Internal cycling of nitrate in soils of a mature coniferous forest. Ecology 73: 1148-1156
Eyre FH (1980) Forest cover types of the United States and Canada. Soc. Am. Foresters, Washington, D.C., U.S.A.
Fahey TJ & Hughes JW (1994) Fine root dynamics in a northern hardwood forest ecosystem, Hubbard Brook Experimental Forest, NH. Journ. Ecology 82: 533-548
Fahey TJ, Battles JJ & Wilson GF (1998) Responses of early successional northern hardwood forests to changes in nutrient availability. Ecol. Monographs 68: 183-212
Federer C (1983) Nitrogen minrealization and nitrification: Depth variation in four New England soils. Soil Sci. Soc. Am. Journ. 47: 1008-1014
Federer CA (1984) Organic matter and nitrogen content of the forest floor in even-aged northern hardwoods. Can. Journ. For. Res. 14: 763-767
Fisk MC & Fahey TJ (1990) Nitrification potentials in organic horizons following clearfelling of northern hardwood forests. Soil Biol. Biochem. 22: 277-279
Fisk MC & Schmidt SK (1996) Microbial responses to nitrogen additions in alpine tundra soil. Soil Biol. Biochem. 28: 751-755
Fisk MC, Schmidt SK & Seastedt TR (1998) Topographic patterns of above-and belowground production and nitrogen cycling in alpine tundra. Ecology 79: 2253-2266
Flanagan J & VanCleve K (1983) Nutrient cycling in relation to decomposition and organic matter quality in taiga ecosystems. Can. Journ. For. Res. 13: 795-817
Gallardo A & Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol. Biochem. 26: 1409-1415
Hart SC, Nason GE, Myrold DD & Perry DA (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75: 880-891
Hart SC & Stark JM (1997) Nitrogen limitation of the microbial biomass in an old-growth forest soil. Ecoscience 4: 91-98
Hart SC, Binkley D & Perry DA (1997) Influence of red alder on soil nitrogen transformations in two conifer forests of contrasting productivity. Soil Biol. Biochem. 29: 1111-1123
Huffman EWD Jr (1977) Performance of a new carbon dioxide coulometer. Microchemistry Journ. 22: 567-573
Insam H & Domsch KH (1988) Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microb. Ecol. 15: 177-188
Kirkham D & Bartholomew WW (1954) Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci. Soc. Am. Proc. 18: 33-34
Kononova MM (1966) Soil organic matter; its nature, its role in soil formation, and its role in fertility. Pergamon, New York
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM & Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol. Appl. 7: 402-415
Magill AH, Downs MR, Nadelhoffer KJ, Hallet RA & Aber JD (1996) Forest ecosystem response to four years of chronic nitrate and sulfate additions at Bear Brooks Watershed, Maine, U.S.A. For. Ecol. Man. 84: 29-37
McNulty SG, Aber JD & Newman SD (1996) Nitrogen saturation in a high elevation spruce-fir stand. For. Ecol. Man. 84: 109-121
Menzel DW & Vaccaro RF (1964) The measurement of dissolved organic and particulate carbon in seawater. Limnol. Oceanogr. 9: 138-142
Mou P, Fahey TJ & Hughes JW (1993) Effects of soil disturbance on vegetation recovery and nutrient accumulation following whole-tree harvest of a northern hardwood ecosystem. Journ. Appl. Ecology 30: 661-675
Nadelhoffer KJ, Aber JD & Melillo JM (1983) Leaf-litter production and soil organic matter dynamics along a nitrogen-availability gradient in Southern Wisconsin (U.S.A.) Can. Journ. For. Res. 13: 12-21
Newman EI (1985) The rhizosphere-C sources and microbial populations. In: Fitter AH (Ed.) Ecological Interactions in Soil (pp 107-121). Blackwell, London
Nohrstedt H, Arnebrant K, Bååth E & Söderström B (1989) Changes in C content, respiration rate, ATP content, and microbial biomass in N fertilized pine forest soils in Sweden. Can. Journ. For. Res. 19: 323-328
Pastor J, Aber J, McClaugherty C & Melillo J (1984) Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65: 256-268
Paul EA & Johnson RL (1977) Microscopic counting and ATP measurement in determining microbial growth in soil. Appl. Environ. Microbiol. 34: 263-269
Powlson DS, Brookes PC & Jenkinson DS (1987) Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 19: 159-164
Prescott CE, Corbin JP & Parkinson D (1992) Immobilization and availability of N and P in the forest floors of fertilized Rocky Mountain coniferous forests. Plant Soil 143: 1-10
Reich PB, Grigal DF, Aber JD & Gower ST (1997) Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78: 335-347
Scheu S (1990) Changes in microbial nutrient status during secondary succession and its modification by earthworms. Oecologia 84: 351-358
Schimel DS (1988) Calculation of microbial growth efficiency from 15N immobilization. Biogeochem. 6: 239-243
Schimel DS (1986) Carbon and nitrogen turnover in adjacent grassland and cropland ecosystems. Biogeochem. 2: 345-357
Scott NA, Parfitt RL, Ross DJ & Salt GJ (1998) Carbon and nitrogen transformations in New Zealand plantation forest soils from sites with diferent N status. Can. Journ. For. Res. 28: 967-976
Singh JS, Raghubanshi AS, Singh RS & Srivastava SC (1989) Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338: 499-500
Smith JL & Paul EA (1990) The significance of soil biomass estimates. In: Bollag JM & Stotzky G (Eds) Soil Biochemistry, Vol 6 (pp 357-396). Marcel Dekker, New York
Smolander A, Kurka A, Kitunen V & Mälkönen E (1994) Microbial biomass C and N, and respiratory activity in soil of repeatedly limed and N-and P-fertilized norway spruce stands. Soil Biol. Biochem. 26: 957-962
Söderström B, Bååth E & Lundgren B (1983) Decrease in soil microbial activity and biomass owing to nitrogen amendments. Can. Journ. Microbiol. 29: 1500-1506
Stevenson FJ (1982) Humus chemistry: genesis, composition, reaction. Wiley, New York
Vance ED, Brookes PC & Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19: 703-708
Vitousek PM (1982) Nutrient cycling and nutrient use efficiency. American Naturalist 119: 553-572
Vitousek PM, Gosz JR, Grier CC, Melillo JM & Reiners WA (1982) A comparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol. Mon. 52: 155-177
Vitousek PM & Matson PA (1985) Disturbance, nitrogen availability, and nitrogen losses in an intensively managed loblolly pine plantation. Ecology 66: 1360-1376
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 67: 321-538
Wardle DA & Parkinson D (1990) Comparison of physiological techniques for estimating the response of the soil microbial biomass to soil moisture. Soil Biol. Biochem. 22: 817-823
Wardle DA & Ghani A (1995) A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 27: 1601-1610
Yanai RD (1992) Phosphorus budget of a 70-year-old northern hardwood foerst. Biogeochem. 17: 1-22
Zak DR, Grigal DF, Gleeson S & Tilman D (1990) Carbon and nitrogen cycling during old-field succession: constraints on plants and microbial biomass. Biogeochem. 11: 111-129
Zak DR, Tilman D, Parmenter RR, Rice CW, Fisher FM, Vose J, Milchunas D & Martin CW (1994) Plant production and soil microorganisms in late-successional ecosystems: A continental-scale study. Ecology 75: 2333-2347
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisk, M.C., Fahey, T.J. Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests. Biogeochemistry 53, 201–223 (2001). https://doi.org/10.1023/A:1010693614196
Issue Date:
DOI: https://doi.org/10.1023/A:1010693614196