Abstract
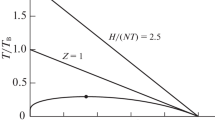
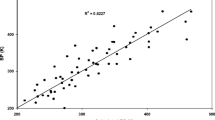
A new corresponding states correlation for the second virial coefficient of nonpolar fluids in terms of the boiling point constants is presented. The scaling constants are the normal boiling point temperature, T bp, which is used to form a dimensionless temperature and the liquid density at the normal boiling point, ρ bp, which is used to form a dimensionless second virial coefficient. The procedure has been examined for a large number of substances including noble gases, diatomic molecules, saturated hydrocarbons up to C8, and a number of aliphatic, aromatic, and cyclic hydrocarbons. The resulting correlation has been applied to predict the equation of state of fluids over the range from the vapor-pressure curve to the freezing curve at various temperatures from the triple point up to the nonanalytical critical region. The equation of state has been applied to reproduce the liquid density of a great number of compounds both in the saturation and compressed states, at temperatures up to 2000 K and pressures up to 10000 bar, within an accuracy of a few percent. In particular we have shown that knowledge of two readily measurable constants is sufficient to determine the pvT surface of pure normal fluids having a variety of structural complexities.
Similar content being viewed by others
REFERENCES
A. Barker and D. Henderson, J. Chem. Phys. 47:4714 (1967).
J. D. Weeks, D. Chandler, and H. C. Andersen, J. Chem. Phys. 54:5237 (1971).
G. Ihm, Y. Song, and E. A. Mason, J. Chem. Phys. 94:3839 (1991).
Y. Song and E. A. Mason, J. Chem. Phys. 93:686 (1990).
Y. Song and E. A. Mason, J. Chem. Phys. 91:7840 (1989).
Y. Song and E. A. Mason, Fluid Phase Equil. 75:105 (1992).
K. S. Pitzer, J. Chem. Phys. 7:583 (1939).
D. Berthelot, Trav. et. Mèm. Bur. Int. Poid et Mes. 13 (1907).
K. S. Pitzer and R. F. Curl, Jr., J. Am. Chem. Soc. 79:2369 (1957).
K. S. Pitzer, D. Z. Lippmann, R. F. Curl, Jr., C. M. Huggins, and D. E. Petersen, J. Am. Chem. Soc. 77:3433 (1955).
R. C. Reid, J. M. Prausnitz, and B. E. Polling, The Properties of Gases and Liquids, 4th ed. (McGraw–Hill, New York, 1987).
A. Boushehri and E. A. Mason, Int. J. Thermophys. 14:685 (1993).
M. H. Ghatee and A. Boushehri, Int. J. Thermophys. 17:945 (1996).
J. H. Dymond and E. B. Smith, The Virial Coefficients of Pure Gases and Mixtures. A Critical Compilation (Oxford University, Oxford, 1980).
M. Michels, J. M. Lupton, T. Wassenaar, and W. de Graaff, Physica 18:121 (1952).
K. Strein, R. N. Lichtenthaler, B. Schramm, and K. Schafer, Ber. Bunsenges. Phys. Chem. 75:1308 (1971).
N. Al-Bizreh and C. J. Wormald, J. Chem. Thermodyn. 10:231 (1978).
E. E. Roper, J. Phys. Chem. 44:835 (1940).
L. E. Kolysko, Z. S. Belousova, T. D. Sulimova, L. V. Mozhginskaya, and V. M. Prokhorov, Russ. J. Phys. Chem. 47:1067 (1973); Zh. Fiz. Khim. 47:1890 (1973).
M. L. McGlashan and C. J. Wormald, Trans. Faraday Soc. 60:646 (1964).
F. M. Tao and E. A. Mason, Int. J. Thermophys. 13:1053 (1992).
N. B. Vargaftik, Handbook of Physical Properties of Liquids and Gases, 2nd ed., English translation (Hemisphere, New York, 1983).
R. B. Stewart and R. T. Jacobsen, J. Phys. Chem. Ref. Data 18:639 (1989).
R. T. Jacobsen, R. B. Stewart, and M. Jahangiri, J. Phys. Chem. Ref. Data 15:735 (1986).
R. B. Stewart, R. T. Jacobsen, and W. Wagner, J. Phys. Chem. Ref. Data 20:917 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eslami, H. Equation of State for Nonpolar Fluids: Prediction from Boiling Point Constants. International Journal of Thermophysics 21, 1123–1137 (2000). https://doi.org/10.1023/A:1026498021221
Issue Date:
DOI: https://doi.org/10.1023/A:1026498021221