Abstract
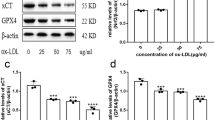
Photodynamic therapy (PDT) is an effective procedure for the treatment of lesions diseases based on the selectivity of a photosensitising compound with the ability to accumulate in the target cell. Atherosclerotic plaque is a suitable target for PDT because of the preferential accumulation of photosensitisers in atherosclerotic plaques. Dendrimers are hyperbranched polymers conjugated to drugs. The dendrimers of ALA hold ester bonds that inside the cells are cleaved and release ALA, yielding PpIX production. The dendrimer 6m-ALA was chosen to perform this study since in previous studies it induced the highest porphyrin macrophage: endothelial cell ratio (Rodriguez et al. in Photochem Photobiol Sci 14:1617–1627, 2015). We transformed Raw 264.7 macrophages to foam cells by exposure to oxidised LDLs, and we employed a co-culture model of HMEC-1 endothelial cells and foam cells to study the affinity of ALA dendrimers for the foam cells. In this work it was proposed an in vitro model of atheromatous plaque, the aim was to study the selectivity of an ALA dendrimer for the foam cells as compared to the endothelial cells in a co-culture system and the type of cell death triggered by the photodynamic treatment. The ALA dendrimer 6m-ALA showed selectivity PDT response for foam cells against endothelial cells. A light dose of 1 J/cm2 eliminate foam cells, whereas less than 50% of HMEC-1 is killed, and apoptosis cell death is involved in this process, and no necrosis is present. We propose the use of ALA dendrimers as pro-photosensitisers to be employed in photoangioplasty to aid in the treatment of obstructive cardiovascular diseases, and these molecules can also be employed as a theranostic agent.








Similar content being viewed by others
Abbreviations
- ALA:
-
5-Aminolevulinic acid
- FBS:
-
Foetal bovine serum
- MTT:
-
(3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide)
- PDT:
-
Photodynamic therapy
- PpIX:
-
Protoporphyrin IX
- PBS:
-
Buffer phosphate
References
Rodriguez, L., Vallecorsa, P. D., Battah, S., Di Venosa, G. M., Mamone, L. A., Saenz, D. A., Gonzalez, M. C., Batlle, A. J., MacRobert, A. J., & Casas, A. G. (2015). Aminolevulinic acid dendrimers in photodynamic treatment of cancer and atheromatous disease. Photochemical and Photobiological Sciences, 14, 1617–1627.
Dougherty, T. J., Kaufman, J. E., Goldfarb, A., Weishaupt, K. R., Boyle, D., & Mittleman, A. (1978). Photoradiation therapy for the treatment of malignant tumors. Cancer Research, 38, 2628–2635.
Agostinis, P., Berg, K., Cengel, K. A., Foster, T. H., Girotti, A. W., Gollnick, S. O., Hahn, S. M., Hamblin, M. R., Juzeniene, A., Kessel, D., Korbelik, M., Moan, J., Mroz, P., Nowis, D., Piette, J., Wilson, B. C., & Golab, J. (2011). Photodynamic therapy of cancer: an update. CA A Cancer Journal for Clinicians, 61, 250–281.
Kennedy, J. C., Marcus, S. L., & Pottier, R. H. (1996). Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. Journal of Clinical Laser Medicine and Surgery, 14, 289–304.
Fukuda, H., Casas, A., Chueke, F., Paredes, S., & Batlle, A. M. C. (1993). Photodynamic action of endogenously synthesized porphyrins from aminolevulinic acid, using a new model for assaying the effectiveness of tumoral cell killing. International Journal of Biochemistry, 25, 1395–1398.
Wen, X., Li, Y., & Hamblin, M. R. (2017). Photodynamic therapy in dermatology beyond non-melanoma cancer: an update. Photodiagnosis and Photodynamic Therapy, 19, 140–152.
Casas, A. (2020). Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: a review. Cancer Letters, 490, 165–173.
Zhou, T., Battah, S., Mazzacuva, F., Hider, R. C., Dobbin, P., & Macrobert, A. J. (2018). Design of bifunctional dendritic 5-aminolevulinic acid and hydroxypyridinone conjugates for photodynamic therapy. Bioconjugate Chemistry, 29, 3411–3428.
Battah, S. H., Chee, C. E., Nakanishi, H., Gerscher, S., MacRobert, A. J., & Edwards, C. (2001). Synthesis and biological studies of 5-aminolevulinic acid-containing dendrimers for photodynamic therapy. Bioconjugate Chemistry, 12, 980–988.
Battah, S., Balaratnam, S., Casas, A., O’Neill, S., Edwards, C., Batlle, A., Dobbin, P., & MacRobert, A. J. (2007). Macromolecular delivery of 5-aminolaevulinic acid for photodynamic therapy using dendrimer conjugates. Molecular Cancer Therapeutics, 6, 876–885.
Kou, J., Dou, D., & Yang, L. (2017). Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget, 8, 81591–81603.
Roy, T., Forbes, T., Wright, G., & Dueck, A. (2015). Burning bridges: Mechanisms and implications of endovascular failure in the treatment of peripheral artery disease. The Journal of Endovascular Therapy, 22, 874–880.
Houthoofd, S., Vuylsteke, M., Mordon, S., & Fourneau, I. (2020). Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis and Photodynamic Therapy, 29, 101568.
Straight, R., Vincent, G., & Hammond, E. (1986). Porphyrin retention and photodynamic treatment of diet induced atherosclerotic lesions in pig. Photodynamic therapy of tumors and other diseases. Padova: Libreria Progetto.
Eldar, M., Yerushalmi, Y., Kessler, E., Scheinowitz, M., Goldbourt, U., Ben Hur, E., Rosenthal, I., & Battler, A. (1990). Preferential uptake of a water-soluble phthalocyanine by atherosclerotic plaques in rabbits. Atherosclerosis, 84, 135–139.
Hamblin, M. R., & Luke Newman, E. (1994). New trends in photobiology. On the mechanism of the tumour-localising effect in photodynamic therapy. The Journal of Photochemistry and Photobiology B Biology, 23, 3–8.
Spokojny, A. M., Serur, J. R., Skillman, J., & Richard Spears, J. (1986). Uptake of hematoporphyrin derivative by atheromatous plaques: Studies in human in vitro and rabbit in vivo. Journal of the American College of Cardiology, 8, 1387–1392.
Rockson, S. G., Lorenz, D. P., Cheong, W. F., & Woodburn, K. W. (2000). Photoangioplasty: An emerging clinical cardiovascular role for photodynamic therapy. Circulation, 102, 591–596.
Jenkins, M. P., Buonaccorsi, G., MacRobert, A., Bishop, C. C. R., Brown, S. G., & McEwan, J. R. (1998). Intra-arterial photodynamic therapy using 5-ALA in a swine model. European Journal of Vascular and Endovascular Surgery, 16, 284–291.
Jenkins, M. P., Buonaccorsi, G. A., Mansfield, R., Bishop, C. C. R., Bown, S. G., & McEwan, J. R. (2000). Reduction in the response to coronary and iliac artery injury with photodynamic therapy using 5-aminolaevulinic acid. Cardiovascular Research, 45, 478–485.
Mansfield, R. J. R., Jenkins, M. P., Pai, M. L., Bishop, C. C. R., Bown, S. G., & McEwan, J. R. (2002). Long-term safety efficacy of superficial femoral artery angioplasty with adjuvant photodynamic therapy to prevent restenosis. British Journal of Surgery, 89, 1538–1539.
De Oliveira Gonçalves, K., Da Silva, M. N., Sicchieri, L. B., De Oliveira Silva, F. R., De Matos, R. A., & Courrol, L. C. (2015). Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. The Analyst, 140, 1974–1980.
Moore, K. J., & Tabas, I. (2011). Macrophages in the pathogenesis of atherosclerosis. Cell, 145, 341–355.
Sengupta, B., Narasimhulu, C. A., & Parthasarathy, S. (2013). Novel technique for generating macrophage foam cells for in vitro reverse cholesterol transport studies. Journal of Lipid Research, 54, 3358–3372.
Collot-Teixeira, S., Martin, J., McDermott-Roe, C., Poston, R., & McGregor, J. L. (2007). CD36 and macrophages in atherosclerosis. Cardiovascular Research, 75, 468–477.
Ledda, A., González, M., Gulfo, J., Díaz Ludovico, I., Ramella, N., Toledo, J., Garda, H., Grasa, M., & Esteve, M. (2016). Decreased OxLDL uptake and cholesterol efflux in THP1 cells elicited by cortisol and by cortisone through 11β-hydroxysteroid dehydrogenase type 1. Atherosclerosis, 250, 84–94.
Ades, E. W., Candal, F. J., Swerlick, R. A., George, V. G., Summers, S., Bosse, D. C., & Lawley, T. J. (1992). HMEC-1: establishment of an immortalized human microvascular endothelial cell line. The Journal of Investigative Dermatology, 99, 683–690.
Tricerri, A., Córsico, B., Toledo, J. D., Garda, H. A., & Brenner, R. R. (1998). Conformation of apolipoprotein AI in reconstituted lipoprotein particles and particle-membrane interaction: Effect of cholesterol. Biochimica et Biophysica Acta (BBA) Lipids and Lipid Metabolism, 1391, 67–78.
Denizot, F., & Lang, R. (1986). Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. The Journal of Immunological Methods, 89, 271–277.
Rodriguez, L., de Bruijn, H. S., Di Venosa, G., Mamone, L., Robinson, D. J., Juarranz, A., Batlle, A., & Casas, A. (2009). Porphyrin synthesis from aminolevulinic acid esters in endothelial cells and its role in photodynamic therapy. The Journal of Photochemistry and Photobiology B Biology, 96, 249–254.
Toledo, J., Esteve, M., Grasa, M., Ledda, A., Garda, H., Gulfo, J., Ludovico, I. D., Ramella, N., & Gonzalez, M. (2016). Data related to inflammation and cholesterol deposition triggered by macrophages exposition to modified LDL. Data in Brief, 8, 251–257.
Demidova, T. N., & Hamblin, M. R. (2004). Photodynamic therapy targeted to pathogens. International Journal of Immunopathology and Pharmacology, 17, 245–254.
Wahl, L., & Kleinman, H. (1998). Tumor-associated macrophages as targets for cancer therapy. Journal of the National Cancer Institute, 90, 1583–1584.
Korbelik, M., & Hamblin, M. R. (2015). The impact of macrophage-cancer cell interaction on the efficacy of photodynamic therapy. Photochemical and Photobiological Sciences, 14, 1403–1409.
Evans, S., Matthews, W., Perry, R., Fraker, D., Norton, J., & Pass, H. I. (1990). Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. Journal of the National Cancer Institute, 82, 34–39.
Hamblin, M. R., Tawakol, A., Castano, A. P., Gad, F., Zahra, T., Ahmadi, A., Stern, J., Ortel, B., Chirico, S., Shirazi, A., Syed, S., & Muller, J. E. (2003). Macrophage-targeted photodynamic detection of vulnerable atherosclerotic plaque. Lasers in surgery: Advanced characterization, therapeutics, and systems XIII (Vol. 4949, p. 466). Bellingham: SPIE.
Schmitt, F., Lagopoulos, L., Käuper, P., Rossi, N., Busso, N., Barge, J., Wagnières, G., Laue, C., Wandrey, C., & Juillerat-Jeanneret, L. (2010). Chitosan-based nanogels for selective delivery of photosensitizers to macrophages and improved retention in and therapy of articular joints. Journal of Controlled Release, 144, 242–250.
Amer, A. O., & Swanson, M. S. (2002). A phagosome of one’s own: A microbial guide to life in the macrophage. Current Opinion in Microbiology, 5, 56–61.
Stafford, J. L., Neumann, N. F., & Belosevic, M. (2002). Macrophage-mediated innate host defense against protozoan parasites. Critical Reviews in Microbiology, 28, 187–248.
Bogdanowicz, D. R., & Lu, H. H. (2013). Multifunction co-culture model for evaluating cell-cell interactions (pp. 29–36). New York: Springer New York.
Zuniga, M. C., Raghuraman, G., & Zhou, W. (2018). Physiologic levels of resistin induce a shift from proliferation to apoptosis in macrophage and VSMC co-culture. Surgery, 163(4), 906–911.
Tanabe, S. I., & Grenier, D. (2009). Endothelial cell/macrophage cocultures as a model to study Streptococcus suis-induced inflammatory responses: RESEARCH ARTICLE. FEMS Immunology and Medical Microbiology, 55, 100–106.
Di Venosa, G. M., Casas, A. G., Battah, S., Dobbin, P., Fukuda, H., MacRobert, A. J., & Batlle, A. (2006). Investigation of a novel dendritic derivative of 5-aminolaevulinic acid for photodynamic therapy. International Journal of Biochemistry and Cell Biology, 38, 82–91.
Casas, A., Battah, S., Di Venosa, G., Dobbin, P., Rodriguez, L., Fukuda, H., Batlle, A., & MacRobert, A. J. (2009). Sustained and efficient porphyrin generation in vivo using dendrimer conjugates of 5-ALA for photodynamic therapy. Journal of Controlled Release, 135, 136–143.
Kawczyk-Krupka, A., Czuba, Z., Szliszka, E., Król, W., & Sieroń, A. (2011). The role of photosensitized macrophages in photodynamic therapy. Oncology Reports, 26, 275–280.
Syed Abdul Rahman, S. N., Abdul Wahab, N., & Abd Malek, S. N. (2013). In vitro morphological assessment of apoptosis induced by antiproliferative constituents from the rhizomes of Curcuma zedoaria. Evidence-Based Complementary and Alternative Medicine. https://doi.org/10.1155/2013/257108
Tewari, K. M., & Eggleston, I. M. (2018). Chemical approaches for the enhancement of 5-aminolevulinic acid-based photodynamic therapy and photodiagnosis. Photochemical and Photobiological Sciences, 17, 1553–1572.
Acknowledgements
Work at CIPYP was supported by grants from CONCET (PIP 0237, to AC) and ANPCyT (PICT 2014-0727, to AC). MC thanks INC for a student fellowship. GC thanks CONICET for a doctoral fellowship. The authors are grateful to Vanina Ripoll for her technical support.
Author information
Authors and Affiliations
Contributions
GD and AC conceived the biological experiments and wrote the main text. MC, DS, GC and GD carried out the biological experiments. MG carried out the isolation of LDL, preparation of OxLDL AM and SB provide the dendrimer 6m-ALA. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Rights and permissions
About this article
Cite this article
Céspedes, M.A., Saénz, D.A., Calvo, G.H. et al. Apoptotic cell death induced by dendritic derivatives of aminolevulinic acid in endothelial and foam cells co-cultures. Photochem Photobiol Sci 20, 489–499 (2021). https://doi.org/10.1007/s43630-021-00025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-021-00025-x