Abstract
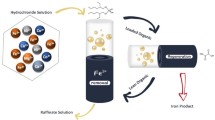
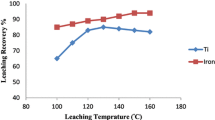
Iron is the major impurity in the pregnant leaching solutions (PLS) derived from heap and atmospheric acid leaching of low-grade laterite ores. The PLS can be considered as a secondary resource for the synthesis of high added-value iron nanomaterials, provided that the iron is selectively separated from the polymetallic PLS solution. In this study, the feasibility of selective iron separation was investigated by applying a solvent extraction technique based on the use of Di-2-ethylhexyl phosphoric acid (D2EHPA) and Tri-butyl phosphate (TBP) extractants. The effect of several parameters such as initial pH and PLS composition was studied to identify the conditions allowing the maximum possible separation of iron with the minimum co-extraction of other valuable metals. It was found that the best separation of Fe could be obtained when the initial pH of the aqueous phase was regulated close to 1.4. The recovery of iron from the loaded organic phase was studied using sulfuric acid as stripping reagent and elemental iron as solid reducing agent. It was found that 90% of Fe(III) could be stripped out by applying the metallic iron (galvanic) stripping.




Similar content being viewed by others
References
Harder J (2018) Metal ore mining in Europe. AT Mineral Processing. https://www.at-minerals.com/en/artikel/. Accessed 25 Jun 2021
European Commission (2020) Raw Materials Information System (RMIS), Policies and definitions. https://rmis.jrc.ec.europa.eu/?page=policies-and-definitions-2d5b5e. 2020). Accessed 3 Jul 2021
Spooren J, Binnemans K, Björkmalm J, Breemersch K, Dams Y, Folens K, González-Moya M, Horckmans L, Komnitsas K, Kurylak W, Lopez M, Mäkinen J, Onisei S, Oorts K, Peys A, Pietek G, Pontikes Y, Snellings R, Tripiana M, Varia J, Willquist K, Yurramendi L, Kinnunen P (2020) Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: technology development trends. Resour Conserv Recycl 160:104919
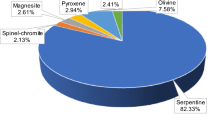
Mystrioti C, Papassiopi N, Xenidis X, Komnitsas K (2018) Counter-current leaching of low-grade laterites with hydrochloric acid and proposed purification options of pregnant solution. Minerals 8:599
Agatzini-Leonardou S, Tsakiridis PE, Oustadakis P, Karidakis T, Katsiapi A (2009) Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores. Miner Eng 22(14):1181–1192
Komnitsas K, Petrakis E, Pantelaki O and Kritikaki A (2018) Column leaching of Greek low-grade limonitic laterites Minerals 8 377
Komnitsas K, Petrakis E, Bartzas G, Karmali V (2019) Column leaching of low-grade saprolitic laterites and valorization of leaching residues. Sci Total Environ 665:347–357
Agrawal A, Kumari S, Sahu KK (2011) Studies on solvent extraction of iron(III) as a step for conversion of a waste effluent to a value added product. J Environ Manag 92:3105–3111
Wang K, Li J, McDonald RG, Browner RE (2013) Characterisation of iron-rich precipitates from synthetic atmospheric nickel laterite leach solutions. Miner Eng 40:1–11
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. Wiley-VCH GmbH and Co, KGaA, Weinheim
Yi X, Huo G, Tang W (2020) Removal of Fe(III) from Ni-Co-Fe chloride solutions using solvent extraction with TBP. Hydrometallurgy 192:105265
Mansur MB (2011) Solvent extraction for metal and water recovery from industrial wastes and effluents. REM: Rem: Rev Esc Minas, Ouro Preto 64(1):051–055
Singh DK, Mishra SL, Singh H (2006) Stripping of iron (III) from the D2EHPA-TBP extract produced during uranium recovery from phosphoric acid by oxalic acid. Hydrometallurgy 81:214–218
Singh DK, Yadav KK, Singh H (2013) Extraction and stripping behavior of iron (III) from phosphoric acid medium by D2EHPA alone and its mixtures with TBP/TOPO. Sep Sci Technol 48:1556–1564
Ghafarizadeh B, Rashchi F (2011) Separation of manganese and iron from reductive leaching liquor of electric arc furnace dust of ferromanganese production units by solvent extraction. J Chem Pet Eng 45:97–107
Ismael MRC, Carvalho JMR (2003) Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Miner Eng 16:31–39
Lee PC, Li CW, Chen JY, Li YS, Chen SS (2011) Dissolution of D2EHPA in liquid-liquid extraction process: Implication on metal removal and organic content of the treated water. Water Res 45:5953–5958
Chandrasekar A, Suresh A, Sivaraman N (2017) Third phase formation in the extraction of Th(NO3)4 by Tri-sec-butyl phosphate: a comparison with Trin-butyl phosphate. Radiochim Acta 105(4):321–328
Principe F, Demopoulos GP (2004) Comparative study of iron (III) separation from zinc sulphate-sulphuric acid solution using the organophosphorous extractant, OPAP and D2EHPA Part I: Extraction. Hydrometallurgy 74:93
Principe F, Demopoulos GP (2005) Comparative study of iron (III) separation from zinc sulphate-sulphuric acid solution using the organophosphorous extractant, OPAP and D2EHPA Part II Stripping. Hydrometallurgy 79:97–109
Riveros PA, Dutrizac JE, Benguerel E, Houlachi G (1998) The recovery of iron from zinc sulphate–sulphuric acid processing solutions by solvent extraction or ion exchange. Miner Process Extr Metall Rev 18:105–145
El-Nadi YA, El-Hefny NE (2010) Removal of iron from Cr-electroplating solution by extraction with di(2-ethylhexyl)phosphoric acid in kerosene. Chem Eng Process 49:159–164
Yu S, Chen J (1989) Stripping of Fe(III) extracted by Di-2-ethylhexyl phosphoric acid from sulfate solutions with sulfuric acid. Hydrometallurgy 22:267–272
Cao X, Zhang T, Zhang W, Lv G (2020) Solvent extraction of Sc(III) by D2EHPA/TBP from the leaching solution of vanadium slag. Metals 10:790
Ma H, Li H, Wu W, Qiao XR (2017) Separation of Fe(III) and Cr(III) from tannery sludge bioleachate using organophosphorus acid extractants. Res Chem Intermed 3:2333–2350
Demopoulos GP, Gefvert DL (1984) Iron (III) removal from base-metal electrolyte solutions by solvent extraction. Hydrometallurgy 12:299–315
Majima H, Izaki T, Sanuki S (1985) Reductive stripping of Fe(III)-loaded D2EHPA with aqueous solutions containing sulfur dioxide. Metall Trans A 16B:187–194
Liu Y, Nam SH, Lee M (2014) Stripping of Fe(III) from the loaded mixture of D2EHPA and TBP with sulfuric acid containing reducing agents. Bull Kor Chem Soc 35(7):2109
Sun J, O’Keefe TJ (2002) An evaluation of steel scrap as a reducing agent in the galvanic stripping of iron from D2EHPA. Miner Eng 15:177–185
Bartzas G, Komnitsas K, Paspaliaris I (2006) Laboratory evaluation of Fe0 barriers to treat acidic leachates. Miner Eng 19(5):505–514
Touomo-Wouafo M, Donkeng-Dazie J, Btatkeu-K BD, Tchatchueng JB, Noubactep C, Ludvík J (2018) Role of pre-corrosion of Fe0 on its efficiency in remediation systems: an electrochemical study. Chemosphere 209:617–622
Hu G, Wu Y, Chen D, Wang Y, Qi T, Wang L (2020) Selective removal of iron(III) from highly salted chloride acidic solutions by solvent extraction using di(2-ethylhexyl) phosphate. Front Chem Sci Eng 15:528–537
Grand View Research Inc (2019) Magnetite Nanoparticles Market Size. Industry Report:2019–2025. https://www.grandviewresearch.com/industry-analysis/magnetite-nanoparticles-market. Accessed 28 Jun 2021
Brumovsky M, Filip J, Malina O, Oborna J, Sracek O, Reichenauer T, Andryskova P, Zboril R (2020) Core–shell Fe/FeS nanoparticles with controlled shell thickness for enhanced trichloroethylene removal. ACS Appl Mater Interfaces 12:35424–35434
Wei X, Viadero RC (2007) Synthesis of magnetite nanoparticles with ferric iron recovered from acid mine drainage: implications for environmental engineering. Colloids and Surfaces A Physicochemical and Engineering Aspects 294(1):280–286
Availability of Data and Material
The authors confirm that the data supporting the findings of this study are available within the article.
Code Availability
Not applicable.
Funding
This research is co-financed by the Greece and the European Union (European Social Fund (ESF)) through the Operational Programme “Human Resources Development, Education and Lifelong Learning” in the context of the project “Reinforcement of Postdoctoral Researchers - 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ).
Author information
Authors and Affiliations
Contributions
C. Mystrioti: investigation, writing, review and editing, validation, and visualization. N. Papassiopi: conceptualization, methodology, and supervision. A. Xenidis: methodology, formal analysis, and supervision.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Mystrioti, C., Papassiopi, N. & Xenidis, A. Selective Recovery of Iron by Solvent Extraction from Ni-Laterite Leach Solutions, as Precursor for the Synthesis of High Added-Value Nanomaterials. Circ.Econ.Sust. 2, 1575–1587 (2022). https://doi.org/10.1007/s43615-021-00094-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43615-021-00094-1