Abstract
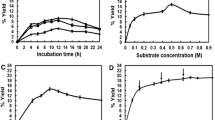
Sucrose phosphorylase (SPase) can transfer the glucosyl group of sucrose to different compounds and has been widely used in industry. To overcome the low thermostability of the sucrose phosphorylase from Leuconostoc mesenteroides ATCC 12291 (LmSP), a method named PROSS was used to construct mutants with increased thermostability. All variants were screened by measuring their residual activities after heating at 50°C. Then, a single point mutant and a combined mutant with improved thermostability and activity were obtained. The half-lives of mutants at 50°C were approximately twice as high as those of the wild type. In addition, 2-O-α-d-glucosylglycerol (αGG) was synthesized by the wild type and the two improved variants, and the reaction conditions were optimized. Under the conditions of glycerol concentration of 3.2 mol/L, sucrose concentration of 1.2 mol/L, and enzyme concentration of 40 U/mL at 37°C for 60 h, the yield of αGG reached the maximum, and the sucrose conversion rate of the wild type, the mutant V23L and the combined mutant V23L/S424R were 62.3%, 70.7% and 76.3%, respectively. In this study, SPase mutants with higher activity and stability were obtained, and achieved high-level production of αGG.





Similar content being viewed by others
References
Goedl C, Schwarz A, Minani A, Nidetzky B. Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: Characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of α-d-glucose 1-phosphate. J Biotechnol. 2007;129:77–86.
Kraus M, Gorl J, Timm M, Seibel J. Synthesis of the rare disaccharide nigerose by structure-based design of a phosphorylase mutant with altered regioselectivity. Chem Commun. 2016;52:4625–7.
Taeyeon K, Cheong Tae K, Jong-Hoon L. Transglucosylation of ascorbic acid to ascorbic acid 2-glucoside by a recombinant sucrose phosphorylase from Bifidobacterium longum. Biotech Lett. 2007;29:611–5.
Sugimoto K, Nomura K, Nishiura H, Ohdan K, Nishimura T, Hayashi H, Kuriki T. Novel transglucosylating reaction of sucrose phosphorylase to carboxylic compounds such as benzoic acid. J Biosci Bioeng. 2007;104:22–9.
Klahn S, Hagemann M. Compatible solute biosynthesis in cyanobacteria. Environ Microbiol. 2011;13:551–62.
Sawangwan T. Glucosylglycerol on performance of prebiotic potential. Function Foods Health Dis. 2015;5:427–36.
Luley-Goedl C, Sawangwan T, Brecker L, Wildberger P, Nidetzky B. Regioselective O-glucosylation by sucrose phosphorylase: a promising route for functional diversification of a range of 1,2-propanediols. Carbohyd Res. 2010;345:1736–40.
Hagemann M. Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev. 2011;35:87–123.
Takenaka F, Uchiyama H. Synthesis of α-d-Glucosylglycerol by α-glucosidase and some of its characteristics. Biosci Biotechnol Biochem. 2000;64:1821–6.
Roder A, Hoffmann E, Hagemann M, Berg G. Synthesis of the compatible solutes glucosylglycerol and trehalose by salt-stressed cells of Stenotrophomonas strains. FEMS Microbiol Lett. 2005;243:219–26.
Klahn S, Steglich C, Hess WR, Hagemann M. Glucosylglycerate: a secondary compatible solute common to marine cyanobacteria from nitrogen-poor environments. Environ Microbiol. 2010;12:83–94.
Goedl C, Sawangwan T, Mueller M, Schwarz A, Nidetzky B. A high-yielding biocatalytic process for the production of 2-O-(α-d-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew Chem. 2010;47:10086–9.
Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review. Biores Technol. 2003;89:17–34.
Cerdobbel A, De Winter K, Desmet T, Soetaert W. Sucrose phosphorylase as cross-linked enzyme aggregate: improved thermal stability for industrial applications. Biotechnol J. 2010;5:1192–7.
Fujii K, Iiboshi M, Yanase M, Takaha T, Kuriki T. Enhancing the thermal stability of sucrose phosphorylase from Streptococcus mutans by random mutagenesis. J Appl Glycosci. 2006;53:91–7.
Cerdobbel A, De Winter K, Aerts D, Kuipers R, Joosten HJ, Soetaert W, Desmet T. Increasing the thermostability of sucrose phosphorylase by a combination of sequence- and structure-based mutagenesis. Protein Eng Des Sel. 2011;24:829–34.
Aerts D, Verhaeghe T, Joosten HJ, Vriend G, Soetaert W, Desmet T. Consensus engineering of sucrose phosphorylase: the outcome reflects the sequence input. Biotechnol Bioeng. 2013;110:2563–72.
Campeotto I, Goldenzweig A, Davey J, Barfod L, Marshall JM, Silk SE, Wright KE, Draper SJ, Higgins MK, Fleishman SJ. One-step design of a stable variant of the malaria invasion protein RH5 for use as a vaccine immunogen. Proc Natl Acad Sci USA. 2017;114:998–1002.
Goldenzweig A, Goldsmith M, Hill SE, Gertman O, Laurino P, Ashani Y, Dym O, Unger T, Albeck S, Prilusky J, Lieberman RL, Aharoni A, Silman I, Sussman JL, Tawfik DS, Fleishman SJ. Automated structure- and sequence-based design of proteins for high bacterial expression and stability. Mol Cell. 2018;70:380.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Hyun-Chang C, Dong-Ho S, Jong-Hyun J, Suk-Jin H, Min-Jung K, Jong-Hoon L, Pahn-Shick C, Hae-Yeong K, Cheon-Seok P. Development of new assay for sucrose phosphorylase and its application to the characterization of Bifidobacterium longum SJ32 sucrose phosphorylase. Food Sci Biotechnol. 2011;20:513–8.
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–303.
Franceus J, Capra N, Desmet T, Thunnissen A. Structural comparison of a promiscuous and a highly specific sucrose 6(F)-phosphate phosphorylase. Int J Mol Sci. 2019;20:3906.
Olsson MHM, Sondergaard CR, Rostkowski M, Jensen JH. PROPKA3: consistent treatment of internal and surface residues in empirical pK(a) predictions. J Chem Theory Comput. 2011;7:525–37.
Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, Baker NA. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007;35:W522–5.
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802.
Phillips JC, Hardy DJ, Maia JDC, Stone JE, Ribeiro JV, Bernardi RC, Buch R, Fiorin G, Henin J, Jiang W, McGreevy R, Melo MCR, Radak BK, Skeel RD, Singharoy A, Wang Y, Roux B, Aksimentiev A, Luthey-Schulten Z, Kale LV, Schulten K, Chipot C, Tajkhorshid E. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J Chem Phys. 2020;153:44130.
Mackerell AD, Feig M, Brooks CL. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25:1400–15.
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–616.
Humphrey WF, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–8.
Reetz MT, Carballeira D, J, Vogel A,. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angewandte Chemie-Int Ed. 2006;45:7745–51.
Tan ZJ, Zhao J, Chen JZ, Rao DM, Zhou WJ, Chen N, Zheng P, Sun JB, Ma YH. Enhancing thermostability and removing hemin inhibition of Rhodopseudomonas palustris 5-aminolevulinic acid synthase by computer-aided rational design. Biotech Lett. 2019;41:181–91.
Gromiha MM, Selvaraj S. Inter-residue interactions in protein folding and stability. Prog Biophys Mol Biol. 2004;86:235–77.
Hait S, Mallik S, Basu S, Kundu S. Finding the generalized molecular principles of protein thermal stability. Proteins-Struct Function Bioinform. 2020;88:788–808.
Haney PJ, Badger JH, Buldak GL, Reich CI, Woese CR, Olsen GJ. Thermal adaptation analyzed by comparison of protein sequences from mesophilic and extremely thermophilic Methanococcus species. Proc Natl Acad Sci USA. 1999;96:3578–83.
Guo JL, Cheng ZY, Berdychowska J, Zhu XN, Wang LL, Peplowski L, Zhou ZM. Effect and mechanism analysis of different linkers on efficient catalysis of subunit-fused nitrile hydratase. Int J Biol Macromol. 2021;181:444–51.
Cheng ZY, Lan Y, Guo JL, Ma D, Jiang SJ, Lai QP, Zhou ZM, Peplowski L. Computational design of nitrile hydratase from Pseudonocardia thermophila JCM3095 for improved thermostability. Molecules. 2020;25:4806.
Britton KL, Baker PJ, Borges KMM, Engel PC, Pasquo A, Rice DW, Robb FT, Scandurra R, Stillman TJ, Yip KSP. Insights into thermal-stability from a comparison of the glutamate-dehydrogenases from pyrococcus-furiosus and thermoccus-litorageis. Eur J Biochem. 1995;229:688–95.
Franceus J, Desmet T. Sucrose phosphorylase and related enzymes in glycoside hydrolase family 13: discovery, application and engineering. Int J Mol Sci. 2020;21:2526.
Bolivar JM, Luley-Goedl C, Leitner E, Sawangwan T, Nidetzky B. Production of glucosyl glycerol by immobilized sucrose phosphorylase: options for enzyme fixation on a solid support and application in microscale flow format. J Biotechnol. 2017;257:131–8.
Acknowledgements
This study was funded by the Key Research and Development Program of China (2021YFC2100102-03), the National Natural Science Foundation of China (32001064). The computational results used in this article were obtained using Interdisciplinary Center for Modern Technologies facilities, NCU, Torun, Poland.
Author information
Authors and Affiliations
Contributions
LLY and YYX: designed the research. LLY, YYX, and YJS: performed the research and analyzed the data. PL: ran the MD simulations and analyzed the data, YYL: wrote the paper. HQY, WS, XZC and YYX: supervised the research work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, L., Peplowski, L., Shen, Y. et al. Enhancing thermostability and activity of sucrose phosphorylase for high-level production of 2-O-α-d-glucosylglycerol. Syst Microbiol and Biomanuf 2, 643–652 (2022). https://doi.org/10.1007/s43393-022-00090-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00090-y