Abstract
This research presents the possibility of producing durable foam glasses from glass cullet using SiC/AlN foaming agent. The foaming agent generated by the SiC and AlN couple results in a more homogeneous microstructure and thus the emergence of foam glasses with better properties compared to the nitride foaming agent used alone in our previous work. The fabricated foam had a crack-free, 3-D cellular structure with macropores whose geometries varied between elliptical-, pentagonal-, and hexagonal-shaped constructions. It also had a lightweight (≥ 0.18 g/cm3), high cold crushing strength (≤ 4.5 MPa), low thermal conductivity (0.09–0.16 W/m K), and contained more than ~ 89 vol.% gas bubbles enclosed between 11 vol.% impervious glass walls. The properties accomplished by the foam prepared in this work conform with the requirements of international standard for commercial glass foams, demonstrating its strong capability to be utilized in potential applications in sustainable buildings and energy efficiency in industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to the depletion of natural resources and the accumulation of huge amounts of solid waste, nowadays recycling of industrial waste and by-products has become a priority and has recently attracted a growing area of attention. The solid waste includes millions of tons of glass cullet which are produced annually. The glass waste is typically polluted by a high proportion of fine organic matter together with metallic and non-metallic particles. Removal of these organic and inorganic inclusions is relatively expensive. As a result, in many cases, the production of articles from glass cullet is a low-profit business. Consequently, the amount of accumulated glass waste is steadily rising in landfills [1,2,3]. The global quantity of glass wastes produced in 2018 was approximately 130 million metric tons (MMT); about 20% (~ 26 MMT) of these glass cullets are normally recycled and the rest of these municipal solid wastes (~ 104 MMT) is increasingly amassed in the landfills, causing severe environmental problems [4, 5]. Dealing with such large quantities of glass cullet is a serious issue that needs to be resolved.
Fortunately, incorporation of waste glass in the production of glass foam has recently shown a great deal of interest in the recycling concept and provided an important way to recycle these municipal solid wastes. Glass foams exhibit impressive combination of properties, such as lightweight (> 0.5 g/cm3), nontoxicity, high porosity content (< 60 vol.%) [3], chemical inertness, reasonable compressive strength values (0.4–6 MPa) [4], high thermal insulation (~ 0.1 W/m K), water- and fire-resistance along with the ease of operation, cutting, and drilling [5, 6]. That is why, in recent years, these foamed materials have been widely used in numerous applications, such as refractory linings, catalyst support, filters for hot gases and molten metals, as well as building materials [7,8,9,10].
In general, glass foam is typically prepared by foaming glass matrices with a pore-forming agent such as silicon carbide, carbon, and carbonates close to the glass softening temperature undergoing viscous flow sintering [11]. Soda-lime glass waste derived from the lapping machine has been utilized in glass foam production in a number of research works using various pore-forming agents; the characteristics of these foam glasses are summed up in Table 1. E. Ercenk has studied the effect of clay additives on the foaming and mechanical characteristics of glass foams prepared from soda-lime glass waste using dolomite as a pore-forming agent [12]; the resulting foams had 0.42–2.3 MPa compressive strength, 0.76–1.4 g/cm3 bulk density, and 33–62% apparent porosity in the temperature range 1000–1075 ℃. Recently, our research team has paid more attention to the reuse of landfilled glass cullet in the production of glass foams, which contributes to preserving the environment and providing an added value to this waste. During the past years, Ewais et al. have synthesized foam glasses with 0.65–2.48 MPa CCS, ≤ 0.5 g cm−3 bulk density, and 0.09–0.106 W/m K thermal conductivity starting with soda-lime glass waste along with 2.5–7.5 wt.% AlN foaming agent [13]. Replacement of AlN by SiC in the previous work has led to emergence of glass foam with lower bulk density and higher porosity; however, the refractoriness of the foamed glass obtained by SiC was 50 ℃ lower than that of the glass foam obtained by AlN [14].
In this study, we were able to further improve the physico-mechanical characteristics of glass foams produced from soda-lime glass waste using SiC/AlN couple as a foaming agent rather than a single AlN or SiC. The use of SiC/AlN couple resulted in a more homogeneous microstructure and thus the emergence of a glass foam with better physico-mechanical and thermal characteristics (comparable CCS and BD to those obtained by SiC; as well as comparable refractoriness to those obtained by AlN) compared to the nitride and carbide foaming agents when used separately (please see Table 1). In addition, physical and mechanical characteristics of the international standard of the commercial glass foams are given in Table 1 to easily compare foam values obtained in this work with commercial foam products [15, 16]. The properties accomplished by the glass foam produced in this work are in line with the requirements of the international standard for commercial glass foams, demonstrating their strong capability to be used in potential applications in sustainable buildings and energy efficiency in industry.
2 Materials and experimental procedure
2.1 Materials
The soda-lime glass waste derived from the lapping machine was provided by a municipal recycling company, Cairo, Egypt. A detailed chemical analysis of this glass waste was presented in our previous research work [13]; it composed mainly of SiO2 (71.6 wt.%), Na2O (13.5 wt.%), CaO (9 wt.%), MgO (3.87 wt.%) together with a small amount of Al2O3, Fe2O3, SO3 and K2O (about ~ 1.71 wt.%). High purity AlN and SiC raw materials were supplied by Hong Kong Tepu Refractory Co., Limited, China. Particle size distribution (PSD) results of raw material powders utilized in this study are listed in Table 2. According to this table, 90 wt.% of SiC and AlN powders comprised of particles with a diameter less than 1 and 1.3 µm, respectively.
2.2 Experimental procedure
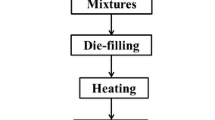
Typical experimental procedures were schematically outlined in Fig. 1. At first, the waste glass was crushed with a small crushing machine. The crushed glass was then ground in a planetary ball mill (weight ratio of glass:zirconia balls = 1:2) at 350 rpm for 90 min. The milled glass powder was subsequently sifted through a 50-mesh sieve. Three batch compositions (GF2.5–7.5) were manufactured from waste glass powders and SiC/AlN foaming agents according to the nominal compositions displayed in Table 3. The quantity of foaming agent was successively added in equal parts of AlN and SiC. For example, to add 2.5 wt.% AlN/SiC, 1.25 wt.% of each material was singly and successively added to the designed batch composition. These batches were dry-blended in a planetary ball mill for 30 min, then dry-molded in cylindrical stainless steel moulds (interior surface coated with BN). The stainless-steel moulds were then placed in a muffle-furnace and heat-treated at 850–950 ℃ with 5 ℃/min heating rate and 30 min retention time at the foaming temperature. After slow cooling to ambient temperature, demolding, cutting and finishing the sintered briquettes, 37 cm3 glass foam briquettes were obtained (Diameter = 3.07 cm; Height = 5 cm), as shown in schematic diagram in Fig. 1. The physical, mechanical and thermal characteristics of the resulting glass foams have been duly studied as a function of the foaming temperature and the pore-forming agent content. To avoid errors in the results, the measurement was carried out three times and the results were only accepted if the difference between the three values was less than 1.5%.
2.3 Characterization
The bulk density (BD) of the resulting cellular glass foams was measured by the simple equation, BD = W/V, where W: weight of glass foam and V: volume of glass foam. The relative density (RD) was estimated by RD = BD/GFD, where GFD is the true density of glass foam powders = 2.521–2.523 g/cm3. From the relative density value, the total porosity (Ptotal) was determined using the following equation, Ptotal = 1−RD [17, 18]. The mineralogical composition of the synthesized glass foams was investigated using SHIMADZU X-ray diffraction machine (Model: XRD-7000, Japan, Cu kα radiation (λ = 1.5406 Å), 2-theta range from 5 to 70o). The microstructure and morphology of the prepared glass foams were examined by means of a field-emission scanning electron microscope (FESEM, Quanta FEG 250, Holland). The exact cellularity (number of pores per inch, PPI) was estimated by counting the number of pores intersected by a straight line (1”) on the obtained photomicrographs [19]. The FESEM images were uploaded on the ImageJ software which automatically analyzed them giving the pore diameters of the obtained foams. This software is a free online application accessible to all researchers. The compression resistance (cold crushing strength) of the glass foam specimens was measured using Shimadzu testing machine at 1.3 mm/min strain rate (Model: UH-F 1000 KN, Japan). Thermal conductivity, thermal diffusivity and specific heat capacity were measured by the Hot Desk machine (Model: 2500 s, Sweden) at room temperature in normal atmosphere. Particle size distribution (PSD) of raw material powders was investigated by laser diffraction using a Fritsch laser diffractometer (model: Analysette 22 NanoTec). Before PSD measurement, the materials were precisely dispersed via ultrasonication for 1 min using 24 kHz Hielscher device (model: UP400S).
3 Results and discussion
The optimal foaming temperature of soda-lime glass waste with SiC/AlN foaming agent was found experimentally between 850 and 950 ℃. In this temperature range, SiC and AlN oxidize according to Eqs. (1, 2) [20,21,22] releasing gas bubbles through the low-viscosity glass medium. These gases are entrapped through the viscous glass medium leading to expanding the glass matrix giving rise to high-quality cellular materials. The physical, mechanical, thermal, and microscopic properties of the resulting foams have been precisely studied, and the results are presented in subsequent sections.
3.1 Microstucture
Figures 2, 3, and 4 display the FESEM photomicrographs of the resulting “GF2.5–7.5” glass foams sintered at 850, 900, and 950 ℃, respectively. The obtained foams were crack-free and they presented predominantly closed porosity with different geometries that varied between hexagonal-, elliptical-, and pentagonal-shaped structures. FESEM photomicrographs were analyzed using ImageJ software and the variations of mean pore diameter and exact cellularity with the sintering temperature and foaming agent content are depicted in Figs. 5 and 6, respectively. The obtained results showed a good compatibility and a reciprocal relationship between the mean pore diameter and cellularity, where the former increased when the latter decreased and vice versa. This inverse relationship between average pore diameter and microcellularity is a logic, where the number of pores per inch (exact cellularity) diminishes as the pores grow larger. As shown in Fig. 6, the average cellularity of the three foams “GF2.5–7.5” at 850 ℃ was approximately 43 ± 20 PPI, a value which declined to 21 ± 10 and 14 ± 6 PPI at 900 and 950 ℃, respectively. In contrast, the average pore diameter of the foams varied from 0.46 to 1.2 mm at 850 ℃; it went up to 1.355 ± 0.545 and 1.925 ± 0.625 mm at 900 and 950 ℃, respectively. Such increase in the pore diameter with the sintering temperature could be explained in terms of a decrease in the glass viscosity at a higher sintering temperature and the increase in gas pressure in the pores which, in turn, force the pores to develop and the entire glass to expand further. Regardless of the sintering temperature, the mean pore diameter of the produced foams increased with the increase in foaming agent content from 2.5 to 5.0 wt.%, and subsequently decreased after increasing the SiC/AlN content above 5 wt.%. This can be explained as follows: (1) when the foaming agent content is low (2.5 wt.%), a small number of gases is evolved leading to small pore diameter and low porosity (2) The average aperture size increased when the foaming agent content increased to 5 wt.% due to the release of more gases, and coalescence between adjacent pores, a phenomenon that forced the pores to grow larger [7, 23,24,25] (3) Beyond 5 wt.% foaming agent, The glass viscosity enhancement occurred by incorporation of the oxidation products of the foaming agent (Al2O3 and SiO2) seems to be more responsible for the reduced bubble size. A similar trend for the variation of the mean pore diameter with the foaming agent content was observed in several previous works. In light of these findings, the sintering temperature and the amount of pore-forming agents are the primary factors controlling the porosity variation in the final cellular foam products.
3.2 Phase composition evolution
Powder XRD patterns of glass foam samples “GF2.5–7.5” fired at 850, 900, and 950 ℃ were displayed in Fig. 7a, b, c, respectively. The resulting foams had an amorphous nature with a wide halo in the 2θ range from 15 to 35° which is characteristic for the amorphous silica with silanol group (Si–OH) [26, 27]. Nonetheless, partially crystalline phases with very low-intensity diffraction peaks were observed in these glass foams, such as: (1) cristobalite phase (SiO2, at 2θ = 21.72°, #JCPDS#01-082-0512) that was mainly detected in “GF2.5” specimen (2) diopside phase (CaMg(SiO3)2, at 2θ = 29.92 and 35.58°, #JCPDS#00–017-0318) which was dectected in “GF5.0–7.5” specimens. Diopside and cristobalite crystallization was reported in a number of glass foams produced from glass cullet and various foaming agents [7, 12, 28,29,30,31].
3.3 Densification parameters
The densification parameters reflect what happened for the microstructure of the produced foams during sintering. Figures 8 and 9, respectively, show the dependence of bulk density and total porosity of the fabricated foams on the sintering temperature and foaming agent content. Apparently, the bulk density decreased throughout the studied temperature range, and contrarily the total porosity increased with increasing SiC/AlN content from 2.5 to 5 wt.%. This can be easily explained in terms of the formation of larger quantities of gas bubbles as the amount of foaming agent increases [28]. With the SiC/AlN content increase beyond 5 wt.%, a reverse trend occurred, where the density increased and the porosity decreased, featuring V and Ʌ-shaped trends, respectively. The decrease in porosity at the high SiC/AlN content (< 5 wt.%( was attributed to the generation of excessive gas bubbles, breakdown of the struts, and collapse of the structure. At 850 ℃, foamed samples expanded slightly to 3.5 times compared to the green body, giving rise to foam with slightly high density (0.35 ± 0.5 g/cm3) and low porosity (89 ± 2 vol.%), suggesting that the viscosity at this foaming temperature was still relatively high. When the foaming temperature increased to 900–950 ℃, foamed samples were expanded clearly to 5–5.5 times compared to the green body, resulting in foam with quite low density (0.25 ± 0.01 g/cm3 at 900 ℃ & 0.19 ± 0.01 g/cm3 at 950 ℃) and high porosity (92 ± 0.5 vol.% at 900 ℃ & 93.5 ± 0.5 vol.% at 950 ℃). The most efficient foaming effect was registered for samples containing 5 wt.% SiC/AlN, where GF5.0–900 has expanded to around 5 times compared to the green body, registering 92 vol.% porosity, 0.24 g/cm3 bulk density, 12 PPI cellularity, and 1.91 mm pore diameter, whereas GF5.0–950 specimen has expanded to about 5.5 times, recording 94 vol.% porosity, 0.18 g/cm3 bulk density, 10 PPI cellularity, and 2.54 mm pore diameter. Based on the densification results, the best sintering temperature was 900 to 950 ℃, and the optimal foaming content was ranged from 2.5 to 5.0 wt.%.
3.4 Cold crushing strength (CCS)
The variation in the CCS of the resulting foams as a function of sintering temperature and SiC/AlN content is illustrated in Fig. 10. The CCS values of the resulting foamed specimens correlated well with their bulk density values. The foamed specimens at 850 ℃ demonstrated the highest CCS values at the expense of their elevated densities. The glass foams sintered at 850 ℃ had compressive strength values between 1.5 and 4.5 MPa, whereas the CCS values decreased to 0.7–1.7 MPa and 0.3–0.8 MPa at 900 and 950 ℃, respectively. Such decrease in the CCS values corresponds to the porosity enhancement and density decline at higher sintering temperatures, since the compressive strength of porous ceramics is basically dependent on the ceramic particles around pores (effective load-bearing struts) which decrease with the increase in porosity at higher sintering temperatures [32, 33]. These findings are in agreement with the reported data in references [13, 34]. The obtained properties for the waste-derived foams synthesized in this work compare well with those displayed by commercially available glass foams in terms of bulk density (0.18–0.4 g/cm3), total porosity (89–94 vol.%), and compressive strength (0.3–4.5 MPa) [11].
3.5 Thermal conductivity
Figure 11 shows the variation of the thermal conductivity of the produced foams with the sintering temperature and SiC/AlN content. The thermal conductivity of glass foams had an inverse proportion with porosity as well as aperture size and homogeneity [35]. This means that the thermal insulation of the glass foams is improved with the increase in porosity and aperture size provided that the pores are uniformly distributed and orderly arranged since the less evenly distributed pores in the glass foam, the smaller the thermal resistance and, consequently, the worse the thermal insulation. Depending on foaming agent content as well as foaming temperature, thermal conductivity of the resulting glass foams ranged between 0.09 and 0.16 W/m K. These values are comparable to those obtained for the foamed materials prepared from the soda-lime glass waste and AlN foaming agent [13].
4 Conclusions
High-quality foam glasses were successfully manufactured from soda-lime glass waste combined with SiC/AlN pore-forming agent via viscous flow sintering at 850–950 ℃. The resulting foams were characterized physically, mechanically, and thermally. The experimental results are set out below:
-
1.
The foam glasses had a 3-D cellular structure with macropores of elliptical, pentagonal, and hexagonal geometries,
-
2.
The glass foam specimens expanded to around 5 times at 900 ℃ compared to the green body, registering 92 ± 0.5 vol.% porosity, 0.25 ± 0.01 g/cm3 bulk density, 21 ± 10 PPI cellularity, 1.355 ± 0.545 mm mean pore diameter, 0.1 W/m–K thermal conductivity, and ~ 0.7–1.7 MPa CCS,
-
3.
The physico-mechanical and thermal properties achieved by the specimens foamed at 900 ℃ are consistent with the requirements of the international standard for commercial glass foams, demonstrating its strong capability to be utilized for potential applications in sustainable buildings and energy efficiency in industry as lining or lightweight packing material.
References
A.A.M. El-Amir, M.A.A. Attia, M. Newishy, T. Fend, E.M.M. Ewais, Aluminium dross/soda lime glass waste-derived high-quality glass foam. J. Mater. Res. Technol. 15, 4940–4948 (2021). https://doi.org/10.1016/j.jmrt.2021.10.085
O.V. Suvorova, N.K. Manakova, D.V. Makarov, Use of bulk industrial wastes in the production of glass foam materials. Glas. Ceram. 77, 384–389 (2021). https://doi.org/10.1007/s10717-021-00312-0 (English Transl. Steklo i Keramika)
I. Bessonov, A. Zhukov, E. Shokodko, A. Chernov, Optimization of the technology for the production of foam glass aggregate. E3S Web Conf (2020). https://doi.org/10.1051/e3sconf/202016414016
I.J. Harder, Glass recycling—current market trends—recovery, recovery. (2018). https://www.recovery-worldwide.com/en/artikel/glass-recycling-current-market-trends_3248774.html (accessed 24 May 2021).
US EPA, Textiles: material-specific data | facts and figures about materials, waste and recycling | US EPA, US EPA. (2018) 1. https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/glass-material-specific-data (accessed 24 May 2021)
E. Bernardo, R. Cedro, M. Florean, S. Hreglich, Reutilization and stabilization of wastes by the production of glass foams. Ceram. Int. 33, 963–968 (2007). https://doi.org/10.1016/j.ceramint.2006.02.010
S. Arcaro, B.G. De Oliveira Maia, M.T. Souza, F.R. Cesconeto, L. Granados, A.P.N. De Oliveira, Thermal insulating foams produced from glass waste and banana leaves. Mater. Res. 19, 1064–1069 (2016). https://doi.org/10.1590/1980-5373-MR-2015-0539
L.P. AG Fedorov, Glass foams: formation, transport properties, and heat, mass, and radiation transfer, J. Non. Cryst. Solids. 311 (2002) 154–173. https://www.sciencedirect.com/science/article/pii/S0022309302013765 (accessed 24 May 2021).
E. Bernardo, F. Albertini, Glass foams from dismantled cathode ray tubes. Ceram. Int. 32, 603–608 (2006). https://doi.org/10.1016/j.ceramint.2005.04.019
R. Ji, Z. Zhang, Y. He, L. Liu, X. Wang, Synthesis, characterization and modeling of new building insulation material using ceramic polishing waste residue. Constr. Build. Mater. 85, 119–126 (2015). https://doi.org/10.1016/j.conbuildmat.2015.03.089
M. Scheffler, P. Colombo, Cellular ceramics: structure, manufacturing, properties and applications, 2006. https://books.google.com/books?hl=en&lr=&id=LP6HpxX0A8MC&oi=fnd&pg=PR5&dq=Scheffler+M,+Colombo+P.+Cellular+ceramics:+structure,+manufacturing,+properties+and+applications.+Weinheim,+Germany:+Wiley‐VCH+Verlag+GmbH+%26+Co.%3B+2005&ots=Z8_oK5Q0x5&sig=7HL2ThR (accessed 24 May 2021).
E. Ercenk, The effect of clay on foaming and mechanical properties of glass foam insulating material. J. Therm. Anal. Calorim. 127, 137–146 (2017). https://doi.org/10.1007/s10973-016-5582-8
E.M.M. Ewais, M.A.A. Attia, A.A.M. El-Amir, A.M.H. Elshenway, T. Fend, Optimal conditions and significant factors for fabrication of soda lime glass foam from industrial waste using nano AlN. J. Alloys Compd. 747, 408–415 (2018). https://doi.org/10.1016/j.jallcom.2018.03.039
A.A. El-Amir, M.A. Attia, T. Fend, E. Ewais, Synthesis of impermeable cellular glass foam from soda lime glass waste using SiC foaming agent. Int. J. Mater. Technol. Innov. 2, 1–4 (2022). https://doi.org/10.21608/IJMTI.2022.97130.1038
G. Scarinci, G. Brusatin, E. Bernardo, Glass foams. Cell. Ceram. Struct. Manuf. Prop. Appl (2006). https://doi.org/10.1002/3527606696.ch2g
H. Elkersh, S. El-Haggar, Recycling of lead crystal glass sludge to produce foam glass. Int. J. Environ. Technol. Manag. 18, 448–464 (2015). https://doi.org/10.1504/IJETM.2015.073089
R.C. da Silva, E.T. Kubaski, E.T. Tenório-Neto, M.K. Lima-Tenório, S.M. Tebcherani, Foam glass using sodium hydroxide as foaming agent: study on the reaction mechanism in soda-lime glass matrix. J. Non. Cryst. Solids. 511, 177–182 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.02.003
C. Mugoni, M. Montorsi, C. Siligardi, F. Andreola, I. Lancellotti, E. Bernardo, L. Barbieri, Design of glass foams with low environmental impact. Ceram. Int. 41, 3400–3408 (2015). https://doi.org/10.1016/j.ceramint.2014.10.127
J. Binner, Ceramics foams. Cell. Ceram. Struct. Manuf. Prop. Appl (2006). https://doi.org/10.1002/3527606696.ch2a
W. Fang, L. Hou, Y. Li, Foaming mechanism of SiC in steel slag foamed ceramics. ISIJ Int. 61, 1043–1052 (2021). https://doi.org/10.2355/ISIJINTERNATIONAL.ISIJINT-2020-271
H. Shi, K. Qin Feng, H. Bo Wang, C. Hong Chen, H. Ling Zhou, Influence of aluminium nitride as a foaming agent on the preparation of foam glass-ceramics from high-titanium blast furnace slag. Int. J. Miner. Metall. Mater. 23, 595–600 (2016). https://doi.org/10.1007/s12613-016-1271-7
R. Lebullenger, S. Chenu, J. Rocherullé, O. Merdrignac-Conanec, F. Cheviré, F. Tessier, A. Bouzaza, S. Brosillon, Glass foams for environmental applications. J. Non. Cryst. Solids (2010). https://doi.org/10.1016/j.jnoncrysol.2010.04.050
P.A.M. dos Santos, A.V. Priebbnow, S. Arcaro, R.M. da Silva, D.A.R. Lopez, A.D.A.L. Rodriguez, Sustainable glass foams produced from glass bottles and tobacco residue. Mater. Res. (2018). https://doi.org/10.1590/1980-5373-mr-2018-0452
F.R. Cesconeto, S. Arcaro, B.G. de Oliveira Maia, M.T. Souza, J.B.R. Neto, A.P.N. de Oliveira, Materiais celulares vítreos obtidos via colagem de gel de uma emulsão de óleo vegetal. Rev. Mater. 21, 385–390 (2016). https://doi.org/10.1590/S1517-707620160002.0036
N. Sasmal, M. Garai, B. Karmakar, Preparation and characterization of novel foamed porous glass-ceramics. Mater. Charact. 103, 90–100 (2015). https://doi.org/10.1016/j.matchar.2015.03.007
S. Kumagai, J. Sasaki, Carbon/silica composite fabricated from rice husk by means of binderless hot-pressing. Bioresour. Technol. 100, 3308–3315 (2009). https://doi.org/10.1016/j.biortech.2009.02.001
E.M.M. Ewais, Y.M.Z. Ahmed, A.A.M. El-Amir, H. El-Didamony, Cement kiln dust/rice husk ash as a low temperature route for wollastonite processing. Epa. J. Silic. Based Compos. Mater. 66, 69–80 (2014). https://doi.org/10.14382/epitoanyag-jsbcm.2014.14
H.R. Fernandes, A. Gaddam, D.U. Tulyaganov, J.M.F. Ferreira, Design and synthesis of foam glasses from recycled materials. Int. J. Appl. Ceram. Technol. 17, 64–74 (2020). https://doi.org/10.1111/ijac.13393
J. Bai, X. Yang, S. Xu, W. Jing, J. Yang, Preparation of foam glass from waste glass and fly ash. Mater. Lett. 136, 52–54 (2014). https://doi.org/10.1016/j.matlet.2014.07.028
J. Deubener, R. Brueckner, H. Hessenkemper, Nucleation and crystallization kinetics on float glass surfaces, Glas. Berichte. 65 (1992) 256–266. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=4339071 (accessed 24 May 2021).
E.D. Zanotto, Surface crystallization kinetics in soda-lime-silica glasses. J. Non. Cryst. Solids. 129, 183–190 (1991). https://doi.org/10.1016/0022-3093(91)90094-M
H. Guo, F. Ye, W. Li, X. Song, G. Xie, Preparation and characterization of foamed microporous mullite ceramics based on kyanite. Ceram. Int. 41, 14645–14651 (2015). https://doi.org/10.1016/j.ceramint.2015.07.186
X. Chen, S. Wu, J. Zhou, Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 40, 869–874 (2013). https://doi.org/10.1016/j.conbuildmat.2012.11.072
D.U. Tulyaganov, H.R. Fernandes, S. Agathopoulos, J.M.F. Ferreira, Preparation and characterization of high compressive strength foams from sheet glass. J. Porous Mater. 13, 133–139 (2006). https://doi.org/10.1007/s10934-006-7014-9
Z. Qin, G. Li, Y. Tian, Y. Ma, P. Shen, Numerical simulation of thermal conductivity of foam glass based on the steady-state method. Materials (Basel). 12, 1–14 (2019). https://doi.org/10.3390/ma12010054
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
El-Amir, A.A.M., Attia, M.A.A., Fend, T. et al. Production of high-quality glass foam from soda lime glass waste using SiC-AlN foaming agent. J. Korean Ceram. Soc. 59, 444–452 (2022). https://doi.org/10.1007/s43207-022-00208-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00208-x