Abstract
Study Objectives
To address sleep micro–macro-structures in psychophysiological insomnia (PPI) as denoted by cyclic alternating pattern (CAP), Sleep spindles, and hyperarousal as microstructures and sleep characteristics such as sleep stages’ variables, and heart rate as macrostructures.
Methods
Two statistical populations, with 20 participants in each, are addressed: good sleepers (GS) and patients with psychophysiological insomnia (PPI). The sleep polysomnography (PSG) for one night was performed and sleep macro–micro-structures extraction was implemented for each participant. Cyclic alternating patterns were scored manually and other structures were monitored by the original PSG’s device software. Analytical methods are used to dissect the results.
Result
The findings imply: (a) psychophysiological insomnia is characterized by CAP differences from good sleepers which are associated with hyperarousal; (b) Regarding microstructure, more microarousals in sleep stages caused more number of wake index. (c) The ratio of sleep stages, sleep latency and heart rate as sleep macrostructure are significantly changed. (d) There is no significant difference between PPI and GS groups on spindles length in our research.
Conclusion
Regarding all sleep disorders and especially PPI, CAP variables, EEG arousals, and sleep spindles as microstructures and Total Sleep Time, Sleep Latency, number of waking, REM duration, and Heart Rate as macrostructures were found to be critical for the diagnosis of psychophysiological insomnia The analysis contributes to understanding better approaches in the quantitative specification of psychophysiological insomnia compare to good sleepers.
Similar content being viewed by others
1 Introduction
Sleep disorders such as various types of insomnia are usual disorders in pediatric practice as their global prevalence is up to 3.7% [1]. Insomnia disorder (ID) refers to difficulties in going to or keeping sleep, or getting up early in the morning. The routine daytime outcomes of this complication are tiredness, moodiness, and cognitive difficulties [2, 3]. As it is noted in the third edition of the International Classification of Sleep Disorders (ICSD-3) [4, 5], ID implies frequency of insomnia symptoms for at least three times a week and more than 3 months and it is referred to as a distinct disease rather than as a disease that is solely dependent on other conditions [6]. The increasing prevalence of ID (3.9–22.1%) is likely caused by genetic and psychophysiological factors such as the aging population, significant stress levels, depression, and anxiety in modern communities. Although ID is characterized by significant medical and mental outcomes, its pathophysiology is not known properly [4, 7,8,9,10,11].
Insomnia is taken as an outcome of dysfunctional perceptions, and maladaptive conduct (e.g. spending an abnormally long time in bed and napping during the day), concerns about the consequences of sleep deprivation, physiological, and cognitive arousal. In addition, Insomnia may be the result of the normal sleep inactivation inhibition (de-arousal) process [12]. There are several cognitive distinctions between good sleepers and people who experience psychological insomnia. Undisturbed low arousal (awakening or de-arousal) is a normal sleep process that allows homeostasis and circadian rhythm requirements to promote sleep. However, insomnia is the result of increased arousal caused by an attempt to sleep. The International Classification of Sleep Disorders (ICSD) 2 specifies 11 insomnia subtypes such as psychophysiological, paradoxical, idiopathic and substance-induced insomnia.
Psychophysiological insomnia (PPI) as a subtype of insomnia occurs during stressful conditions. PPI as an instance of independent insomnia has its pathophysiology; is known to be learned insomnia as it results from a conditioning process in which sleep-associated conditions and hyperarousal are engaged. Low-quality sleep in PPI adds to sleep-associated anxiety, which intensifies hyperarousal even further. Through frequent exposure to sleepless nights, patients with PPI get more concerned with ‘good’ sleep [13]. For instance, people who experience insomnia have more frequent changes in sleep stage, longer stage 1 and shorter stages 3 and 4 [14,15,16]; total sleep time(TST), the number of wakes during the sleep time, and sleep efficiency are also could be measured as other macrostructure signs of insomnia.
On the other hand, sleep spindles that appear during sleep are one of the microstructures of detecting insomnia during sleep time (nonrapid eye movement (N-REM) electroencephalographic oscillations). They can be monitored in stages 2, 3, and 4, but they are features of stage 2 [17]. Spindles can be detected from electroencephalogram (EEG) from 11 to 15 Hz and lasting between 0.5 and 2 s [18]. Two functional roles are attributed to Sleep spindles, learning and plasticity [19] and sleep protection by the inhibition of sensory processing [20,21,22]. Patients with insomnia and Good Sleeper(GS) have no significant differences in the number and density of sleep spindles [23]. However, amplitude, frequency, and duration are not investigated and the role of these features in insomnia has not been cleared.
In addition, the EEG wakefulness variation, the cyclic alternating pattern (CAP), can be measured as another microstructure of insomnia. It is characterized by a sequence of transient electrocortical events of non-REM sleep, often lasting 20–40 s, unlike background EEG activity. CAP is a marker of unstable sleep that the absence of CAP (non-CAP) represents a situation of consolidated sleep. Further studies have found a significant correlation between CAP rate (the ratio of CAP time to non-rem sleep time) and subjective assessment of sleep quality or drowsiness. Therefore, sleep-enhancing treatments increase sleep stability by reducing the amount of CAP and increasing non-CAP [24].
Heart rate also is another sleep macrostructure in psychophysiological insomnia which has been identified as a physiological that can affect morbidity and mortality in insomnia disorder. Heart rate decreases during a typical resting during sleep time and the average rate is 40 to 50 beats per minute (bpm) but this depends on several factors. Some studies showed that heart rate variability is higher in insomniac patients and it is connected with sleep latency and it causes lower sleep quality [25].
The purpose of this study is twofold based on 20 patients affected by psychophysiological insomnia. The first objective is to investigate the sleep macrostructures by comparing the clinical sleep characteristics of PPI and GS. Secondly, studying EEG arousal (sleep spindle) and CAP variables as PPI sleep microstructures.
2 Materials and Methods
2.1 Participants
Forty-five participants were included in this study. Patients with Psychophysiological insomnia were selected from Sleep Research Center, Dana Brain Health Institute, and Shiraz University of Medical Sciences. A somnologist interviewed all patients to ensure they meet the diagnostic criteria and psychiatric interviews for each ICSD psychophysiological ID, overnight PSG, Pittsburgh Sleep Quality Index (PSQI), and Insomnia Severity Index (ISI). Good sleeper participants were also included in local advertisements. The control group consisted of participants with no current or past neurological or psychiatric disorders, with total PSQI and ISI scores < 5. Exclusion criteria were neuropsychiatric drug use, pregnancy, other medical, and neurological or psychiatric disorders. This study was supported by the Research Ethics Committees of Institute for cognitive science studies, Tehran University of Medical Sciences and all participants signed written informed consent forms. Meanwhile, one patient with brain injury and three patients with mild/moderate obstructive sleep apnea, were excluded from the study. Lastly, analyses were performed on 16 patients with PPI and 20 healthy participants.
2.2 PSG Recording and Analyses
On the day of the experiment, patients were asked not to drink coffee or tea, eat heavy meals, or smoke. Patients who were dependent on hypnotics such as benzodiazepines were also excluded. Participants attended the Sleep Research Center at 9 pm and fulfillment demographics, ISI, and PSQI questionnaires. PSG evaluation with the SOMNOscreen ™ plus model (Somnomedics, Germany) was performed for at least 8 h, taking into account the participant’s normal sleep habits. Based on international protocols, sleep rooms have been standardized for noise and visual stimuli [26]. Diagnosis of PPI subtypes was primarily according to ICSD-2 [3, 4]. All cases agreed with the diagnostic criteria for ID according to ICSD3 [3, 4]. This is in close agreement with ICSD2. Both include subjective reports of complications related to sleep onset or retention, proper sleep arrangements, and day order. In addition, Symptoms of PPI also appeared at least three times weekly and had to last for at least 3 months in the PPI group. Substance abuse and any other sleep disorders symptoms were not related to PPI’s Symptoms. PPI was assessed by psychiatric interviews, subjective insomnia symptoms, and Total Sleep Time(TST) ≤ 7 h and Sleep Efficiency(SE) < 85% [27] suggest that sleep evaluation parameters (subjective and objective) are consistent in patients with PPI. Moreover, PSQI and ISI scores are listed in Table 1.
2.3 Macro–Micro Structures Analysis
The domino sleep diagnostic software is used to interface and sleep diagnostic according to AASM and further visual inspection was made to examine Macro–Micro Structures validity.
2.3.1 Typical Sleep Variables (Macrostructure)
The following sleep macrostructure variables were monitored: total sleep time (TST), sleep latency, and wake index (number of wakes during sleep time), total length of stage 1, stage 2, and stage 3 + 4 and REM sleep, and average heart rate (HR) during sleep time.
2.3.2 Sleep Spindle (Microstructure)
People who experience insomnia have higher Cortical hyperarousal compared to good sleepers (GS) and it can be associated with an alteration in sleep safety mechanisms, like decreased density or the sleep spindles variation. Also, the sleep spindle has an important role in sleep consolidation [28]. Sleep spindle detection was performed on NREM epochs with no artifacts for C4:M1.
2.3.3 CAP Variables (Microstructure)
CAP is an episodic EEG activity of NREM sleep that lasts 20–40 s and is counted as microstructure. It is highlighted by a series of biphasic cycles. Phase A considered as transient cortical events including arousal, K complex, and delta burst which cause an increase in frequency and amplitude which is recognizable from the EEG background rhythms called Phase B.
According to the ratio of high-voltage slow waves, rapid low voltage rhythms, and low-amplitude fast rhythms, phase A is classified to:
-
1-
subtype A1 (high-voltage slow waves.)
-
2-
subtype A2 (both fast and slow rhythms)
-
3-
subtype A3 (Rapid low voltage rhythms)
The subsequent CAP variables were monitored in this study: CAP time, CAP rate, number and length of CAP sequences, CAP cycle, and Phase A Subtypes (A1, A2, A3) are based on previous studies. The CAP definition, scoring rules and further study regarding CAP’s role in insomnia are described in [29,30,31].
CAP time: total CAP time during NREM sleep
CAP rate: ratio of CAP time to NREM sleep time
2.4 Statistical Evaluation
SPSS 20 was used to analyze data. Continuous variables were presented as means and standard deviations. First, Kolmogorov–Smirnov (K-S) Test analyzed the distribution within populations. Then, based on the result (sig > 0.05) test is not meaningful, therefore the parametric tests are used. Afterward, the difference between two groups is founded with a t-test considered independent measurements for micro–macro structural variables (control: 20 participants; PPI: 16 patients).
3 Results
3.1 Clinical Characteristics of PPI and GS
Table 2 summarizes the clinical characteristics of participants as sleep macrostructures in this study, and Fig. 1 shows a Strobe diagram for the flow of participants. Concerning GS, higher scores are recorded by patients with PPI on the Pittsburgh Sleep Quality Index (PSQI = 11.8) and Insomnia Severity Index (ISI = 15.7). PSG also demonstrated that patients with PPI had shorter total sleep time (PPI = 370 min, GS = 461 min), increased stage I, sleep higher spontaneous micro-arousal (PPI = 76%, GS = 22%), and more wake index (PPI = 16, GS = 4). Furthermore, patients with PPI recorded diminished sleep efficiency (SE) in comparison with GS. In addition, a comparison of HR showed statistically significant differences between the two groups (Table 1, Fig. 2). PPI showed an increased HR in ECG (PPI = 73(7.8), GS = 55.4(6.4), p < 0.01).
3.2 Cap Analysis
Very significant difference in CAP time (p, 0: 0005) and CAP rate (p, 0: 0001), length of CAP sequence (p, 0:001), number of CAP cycles (p, 0:001), number of subtypes A1 (p, 0:001), subtype A2 (p, 0:001) p, 0: 001) and subtype A3 (p, 0: 001). Although there is no significant difference in the number of CAP sequences and the length of the CAP cycle. Figure 3 represents an example of detecting the A and B phases in a PPI patient.
In summary, patients with PPI represent a meaningful increase in CAP rate and subtypes of Phase A (clearly A1 and A2) compared to controls (Table 2).
3.3 Sleep Spindle Analysis
Table 3 shows the characteristics of sleep spindles. The results represent that there are no critical contrasts between two groups (PPI and GS) in all variables in stage 2 and SWS. Moreover, researchers count the sleep spindle’s amplitude as one of their critical variables whereas reports considering the spindle’s frequency divided to slow (∼11–12 Hz) and fast spindles (∼13–14 Hz), but it is difficult to distinguish this result with our data [23, 32]. Figure 4 represents an example of detecting a Sleep Spindle in a PPI patient.
3.4 Correlation
Table 4 indicates a correlation between microstructures and macrostructures in patients with PPI. Results of the Pearson correlation indicated that there is a significant relationship between heart rate, cap rate, PSQI, ISI and sleep efficiency, REM, and N1 stage. Although, there are no significant differences between sleep latency and other variables. Table 5 indicates all features fluctuation in PPI patients compare to GS.
4 Discussion
Insomnia is a common disorder known to adversely affect daytime function. The increasing prevalence of insomnia disorder is likely caused by genetic and psychophysiological factors such as the aging population, significant stress levels, depression, and anxiety in modern community. Some studies indicate a greater prevalence of heterozygous (A/G) VAL/MET polymorphism in patents with insomnia which impairs BDNF activity that is an important correlate of disturbed sleep [45]. Some patients experience “pure” psychophysiological insomnia, which is often associated with mental illness [33]. As a behaviorally conditioned type of sleep disorder, psychophysiological insomnia is specified by internalized sleep-denying associations that continue even though the initial cause of insomnia is long gone.
Psychophysiological insomnia usually originates from long durations of stress in which stressful reflections keep the patient awake. After a few nights, there comes an increased concern about one’s inability to sleep. Then, a vicious cycle emerges in which concern with an inability to sleep keeps the patient awake.
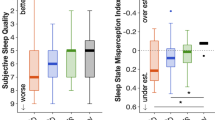
Polysomnography is accompanied by higher sleep latency (longer than 30 min), longer wakefulness after sleep initiates, less than 85 percent efficiency of sleep (i.e. proportion of the total length of sleeping to time in bed), and longer stage I sleep. If an inverse first-night effect is encountered, the patient will know about it; by contrast, a patient reporting sleep state misperception would point to poor sleep quality.
In addition, Psychophysiological insomnia is defined as a state of higher arousal and acquired sleep-preventing association that prevents sleep and leads to inefficient functioning when one is awake [34]. Insomnia should persist for at least 30 days. The physiological arousal might be related to emotions and behaviors that do not satisfy diagnostic criteria for another disorder and the arousal may point to cognitive hypervigilance. The typical symptom for this type of insomnia is termed, ‘racing thoughts’. In this case, the pathologic cycle develops and the more the patient attempts more to go to sleep, the severer his anxiety and agitation becomes. Therefore, the patient will be less capable of sleep initiation.
One likely mechanism for psychophysiological insomnia development is a tendency toward hypervigilance. A relevant model suggests that patients who tend to internalize psychological conflict undergo rising levels of emotional arousal which leads to physiological hyperarousal and denies them of sleep. Furthermore, a recent investigation suggests that the short-term mental health consequences of COVID-19 affect insomniac people and reports of insomnia are significantly increasing [35]. Another research presents the psychophysiological effects of COVID-19 pandemic on social treatment and insomnia [36, 37]. Therefore, psychophysiological insomnia might be the next pandemic sleep disorder that should be more investigated.
The purpose of the study was to investigate on macro- and micro-structures in people who experience psychophysiological insomnia compared to normal. Findings showed that individuals with psychophysiological insomnia were in neurophysiological wakefulness during sleep, patients showed significantly higher sleep latency, spontaneous micro-arousals (MA), number of wakes during the sleep time, and lower sleep efficiency and total sleep time.
The available heart rate measurements are also consistent with the results of previous studies in patients with insomnia [38,39,40]. Comparing the two groups revealed that individuals with psychophysiological insomnia had statistically higher heart rate. In addition, Psychophysiological insomnia appears to be associated with increased levels of CAP and EEG arousals during sleep time and it was observed that CAP/NREM ratio (CAP Rate) is higher in PPI. In our study, the difference between GS and PPI was statistically significant (p < 0.0001).
Some study discovers new methodology to capture CAP automatically and classifies by support vector machine (SVM) and k-nearest (k-NN) which globally, achieves better subtype sensitivities than other approaches [41].
Moreover, sleep spindles in stage 2 are not significantly differed between PPI and GS. Also, the effect of spindles on sleep quality is still unclear and the result is questionable; the literature is rare concerning the different proceeds of the sleep spindle’s specification. Some results indicate that density of sleep spindles is decreased in sleep-deprived condition [42,43,44].
Several study limitations need to be acknowledged. As a CAP detection using manually scoring with SOMNOscreen device software (Domino), there may be inaccuracies associated with CAP subtypes. However, the results show significant differences between two groups based on previous studies [30, 31]. Another limitation is that the sleep spindles have been scored automatically. Therefore, the result may be unsupportive, but this sleep spindle rate is similar to previous studies’ outcomes in patients with PPI [45]. Another limitation of this study is that we did not have access to more patients with PPI because of the COVID-19 pandemic.
In conclusion, it contributed to the determination of ingredient quantitative criteria for PPI according to micro–macro structure (CAP variables, EEG arousals, sleep spindle, and clinical features). Regarding all sleep disorders and especially PPI; CAP variables, EEG arousals and sleep spindles as microstructures and Total Sleep Time, Sleep Latency, number of waking, REM duration, and Heart Rate as macrostructures were found to be critical for the diagnosis of psychophysiological insomnia in investigation based on PSG.
Data availability
Data available on request from the authors.
Abbreviations
- AASM:
-
American Academy of Sleep Medicine
- CAP:
-
Cyclic alternating pattern
- EEG:
-
Electroencephalogram
- GS:
-
Good sleeper
- ICSD:
-
International Classification of Sleep Disorders
- ID:
-
Insomnia disorder
- ISI:
-
Insomnia Severity Index
- MA:
-
Micro-arousals
- N-REM:
-
Nonrapid eye movement
- PPI:
-
Psychophysiological insomnia
- PSG:
-
Polysomnography
- PSQI:
-
Pittsburgh Sleep Quality Index
- REM:
-
Rapid eye movement
- SE:
-
Sleep efficiency
- TST:
-
Total sleep time
References
Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125(6):e1410–8.
Winkelman JW. Insomnia disorder. N Engl J Med. 2015;373(15):1437–44.
Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–58.
Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 suppl):S7–10.
Medicine AAoS. The international classification of sleep disorders:(ICSD-3). American Academy of Sleep Medicine; 2014.
Daley M, Morin CM, LeBlanc M, Grégoire J-P, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64.
Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64.
Garbarino S, Magnavita N, Guglielmi O, et al. Insomnia is associated with road accidents. Further evidence from a study on truck drivers. PLoS ONE. 2017;12(10):e0187256.
Bagherzadeh-Azbari S, Khazaie H, Zarei M, et al. Neuroimaging insights into the link between depression and insomnia: a systematic review. J Affect Disord. 2019;258:133–43.
Emamian F, Khazaie H, Okun ML, Tahmasian M, Sepehry AA. Link between insomnia and perinatal depressive symptoms: a meta-analysis. J Sleep Res. 2019;28(6): e12858.
Murayama T, Ogawa H, Kurebayashi N, Ohno S, Horie M, Sakurai T. A tryptophan residue in the caffeine-binding site of the ryanodine receptor regulates Ca2+ sensitivity. Commun Biol. 2018;1(1):1–12.
Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53(1):215–43.
van de Laar M, Pevernagie D, van Mierlo P, Overeem S. Psychiatric comorbidity and aspects of cognitive coping negatively predict outcome in cognitive behavioral treatment of psychophysiological insomnia. Behav Sleep Med. 2015;13(2):140–56.
Coates TJ, Killen JD, George J, Marchini E, Silverman S, Thoresen C. Estimating sleep parameters: a multitrait-multimethod analysis. J Consult Clin Psychol. 1982;50(3):345.
Hauri P, Fisher J. Persistent psychophysiologic (learned) insomnia. Sleep. 1986;9(1):38–53.
Restifo K, Kupfer J. Persistent psychophysiologic insomnia: preliminary research diagnostic criteria and EEG sleep data. Am J Psychiatry. 1984;141(6):804–5.
De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–40.
Normand M-P, St-Hilaire P, Bastien CH. Sleep spindles characteristics in insomnia sufferers and their relationship with sleep misperception. Neural Plast. 2016. https://doi.org/10.1155/2016/6413473.
Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–65.
Steriade M. Sleep oscillations and their blockage by activating systems. J Psychiatry Neurosci. 1994;19(5):354.
Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–106.
Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85.
Bastien CH, St-Jean G, Turcotte I, Morin CM, Lavallée M, Carrier J. Sleep spindles in chronic psychophysiological insomnia. J Psychosom Res. 2009;66(1):59–65.
Terzano MG, Parrino L, Spaggiari MC, Palomba V, Rossi M, Smerieri A. CAP variables and arousals as sleep electroencephalogram markers for primary insomnia. Clin Neurophysiol. 2003;114(9):1715–23.
Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35(9):1285–91.
Keenan SA. An overview of polysomnography. Handb Clin Neurophysiol. 2005;6:33–50.
Riemann D, Spiegelhalder K, Espie C, et al. Chronic insomnia: clinical and research challenges–an agenda. Pharmacopsychiatry. 2011;44(01):1–14.
Baglioni C, Regen W, Teghen A, et al. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18(3):195–213.
Terzano MG, Parrino L, Smerieri A, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2002;3(2):187–99.
Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev. 2000;4(1):101–23.
Terzano M, Mancia D, Salati M, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985;8(2):137–45.
Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31.
Taylor LM, Espie CA, White CA. Attentional bias in people with acute versus persistent insomnia secondary to cancer. Behav Sleep Med. 2003;1(4):200–12.
Walsh NP, Halson SL, Sargent C, et al. Sleep and the athlete: narrative review and 2021 expert consensus recommendations. Br J Sports Med. 2021;55(7):356–68.
Wauquier A, Aloe L, Declerck A. K-complexes: are they signs of arousal or sleep protective? J Sleep Res. 1995;4(3):138–43.
Sahebi A, Abdi K, Moayedi S, Torres M, Golitaleb M. The prevalence of insomnia among health care workers amid the COVID-19 pandemic: an umbrella review of meta-analyses. J Psychosom Res. 2021;149: 110597.
Clemente-Suárez VJ, Dalamitros AA, Beltran-Velasco AI, Mielgo-Ayuso J, Tornero-Aguilera JF. Social and psychophysiological consequences of the COVID-19 pandemic: an extensive literature review. Front Psychol. 2020;11:3077.
Bonnet MH, Arand D. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–5.
Covassin N, de Zambotti M, Sarlo M, Tona GDM, Sarasso S, Stegagno L. Cognitive performance and cardiovascular markers of hyperarousal in primary insomnia. Int J Psychophysiol. 2011;80(1):79–86.
Oh DY, Park SM, Choi SW. Daytime neurophysiological hyperarousal in chronic insomnia: a study of qEEG. J Clin Med. 2020;9(11):3425.
Machado F, Sales F, Santos C, Dourado A, Teixeira C. A knowledge discovery methodology from EEG data for cyclic alternating pattern detection. BioMed Eng OnLine. 2018;17(1):1–23.
Knoblauch V, Martens WL, Wirz-Justice A, Cajochen C. Human sleep spindle characteristics after sleep deprivation. Clin Neurophysiol. 2003;114(12):2258–67.
De Gennaro L, Ferrara M, Bertini M. Effect of slow-wave sleep deprivation on topographical distribution of spindles. Behav Brain Res. 2000;116(1):55–9.
Curcio G, Ferrara M, Pellicciari MC, Cristiani R, De Gennaro L. Effect of total sleep deprivation on the landmarks of stage 2 sleep. Clin Neurophysiol. 2003;114(12):2279–85.
Zaki NFW, Saleh E, Elwasify M, et al. The association of BDNF gene polymorphism with cognitive impairment in insomnia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:253–64. https://doi.org/10.1016/j.pnpbp.2018.07.025.
Acknowledgements
The authors would like to acknowledge the faculty of medical science and technology, Shiraz University of Medical Sciences, the Dana Brain Institute, Institute for cognitive science, and university of Shahid Beheshti for their support and contribution to this study.
Author information
Authors and Affiliations
Contributions
All authors have seen and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have no known conflict of interest to disclose.
Ethical Approval
The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Approval was obtained from the ethics committee of University of Tehran, Institute of cognitive science. (No: IR.UT.IRICSS.REC.1401.021); Access Link: https://ethics.research.ac.ir/ProposalCertificateEn.php?id=282514&Print=true&NoPrintHeader=true&NoPrintFooter=true&NoPrintPageBorder=true&LetterPrint=true.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghermezian, A., Nami, M., Shalbaf, R. et al. Sleep Micro–Macro-structures in Psychophysiological Insomnia. PSG Study. Sleep Vigilance 7, 55–63 (2023). https://doi.org/10.1007/s41782-023-00228-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41782-023-00228-5