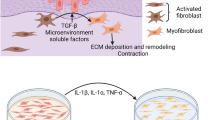
Abstract
Diabetic foot ulcers are one of the common secondary complications associated with diabetes and are characterized by delayed or absence of healing. Of the several factors contributing to poor healing, chronic low-grade inflammation significantly worsens wound healing resulting in adverse outcomes. As the innate immune system plays a vital role in wound healing, a closer look at their alterations may provide insights into developing novel treatment strategies to promote healing. In this review, we discuss the role of the innate immune system in driving chronic inflammation both at the wound site and at the systemic level, resulting in poor healing outcomes. Specifically, we highlight the findings from preclinical and clinical studies that describe the dysregulations of the innate system at the cellular and molecular level and how they contribute to low-grade chronic inflammation in wounds. Our review of the literature shows that preexisting low-grade inflammation, which is associated with altered myeloid cell phenotype and function, is key to impaired wound healing responses in individuals with diabetes. Hence, we suggest that modulating circulating myeloid cell function and low-grade chronic inflammation could be a helpful strategy in promoting diabetic foot ulcer healing.

Similar content being viewed by others
Notes
Wounds that fail to heal within normal timeframe of healing (3–4 weeks).
Process of deterioration with age.
Defense response mounted by the immune system to heal an injury or clear foreign bodies.
Engulfment of dead cells by phagocytic immune cells.
Refers to cells undergoing apoptosis (programmed cell death).
A cellular process of engulfment of particles larger than 500 nm via cellular membrane.
Lack of adequate oxygen.
Condition of elevated blood sugar.
Chemoattractant proteins secreted by cells to direct cell movement and migration.
Proteins secreted by immune cells for cell signaling.
Process of formation of new blood vessels.
Process of formation of blood vessels by de novo production of endothelial cells.
Refers to hydrolysis of proteins or peptides into soluble products.
Process of forming neutrophil extracellular traps.
Transplantation of cells or tissues of an individual from one part of the body to another.
Ability to modulate immune cell activity.
References
Clayton W Jr, Elasy TA (2009) A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabet 27:52–58. https://doi.org/10.2337/diaclin.27.2.52
Bandyk DF (2018) The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg 31:43–48. https://doi.org/10.1053/j.semvascsurg.2019.02.001
Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J (2005) The global burden of diabetic foot disease. Lancet 366:1719–1724. https://doi.org/10.1016/S0140-6736(05)67698-2
Prompers L, Schaper N, Apelqvist J et al (2008) Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. Eurodiale Study Diabetol 51:747–755. https://doi.org/10.1007/s00125-008-0940-0
Abbott CA, Carrington AL, Ashe H et al (2002) The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 19:377–384. https://doi.org/10.1046/j.1464-5491.2002.00698.x
Zhang P, Lu J, Jing Y et al (2017) Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis †. Ann Med 49:106–116. https://doi.org/10.1080/07853890.2016.1231932
Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376:2367–2375. https://doi.org/10.1056/NEJMra1615439
Singh N (2005) Preventing foot ulcers in patients with diabetes. JAMA 293:217. https://doi.org/10.1001/jama.293.2.217
Vibha SP, Kulkarni MM, KirthinathBallala AB et al (2018) Community based study to assess the prevalence of diabetic foot syndrome and associated risk factors among people with diabetes mellitus. BMC Endocr Disord 18:43. https://doi.org/10.1186/s12902-018-0270-2
Cm Z, Dp O et al (2017) A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. https://doi.org/10.1111/iwj.12600
Vanlerberghe B, Devemy F, Duhamel A et al (2014) Traitement chirurgical conservateur du mal perforant plantaire en regard des têtes de métatarsiens chez le diabétique. Étude rétrospective cas-témoins. Annal Chirur Plast Esthét 59:161–169. https://doi.org/10.1016/j.anplas.2013.07.008
Narres M, Kvitkina T, Claessen H et al (2017) Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: A systematic review. PLoS ONE 12:e0182081. https://doi.org/10.1371/journal.pone.0182081
Almaraz MC, González-Romero S, Bravo M et al (2012) Incidence of lower limb amputations in individuals with and without diabetes mellitus in Andalusia (Spain) from 1998 to 2006. Diabetes Res Clin Pract 95:399–405. https://doi.org/10.1016/j.diabres.2011.10.035
Trautner C, Haastert B, Spraul M et al (2001) Unchanged incidence of lower-limb amputations in a German City, 1990–1998. Diabetes Care 24:855–859. https://doi.org/10.2337/diacare.24.5.855
Prompers L, Huijberts M, Apelqvist J et al (2007) High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 50:18–25. https://doi.org/10.1007/s00125-006-0491-1
Huang YY, Lin CW, Yang HM et al (2018) Survival and associated risk factors in patients with diabetes and amputations caused by infectious foot gangrene. J Foot Ankle Res 11:1–4. https://doi.org/10.1186/s13047-017-0243-0
Morbach S, Furchert H, Gröblinghoff U et al (2012) Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 35:2021. https://doi.org/10.2337/dc12-0200
Moulik PK, Mtonga R, Gill GV (2003) Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 26:491–494. https://doi.org/10.2337/diacare.26.2.491
Huang Z-H, Li S-Q, Kou Y et al (2019) Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta-analysis. Int Wound J 16:1373. https://doi.org/10.1111/iwj.13200
Nussbaum SR, Carter MJ, Fife CE et al (2018) An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 21:27–32. https://doi.org/10.1016/j.jval.2017.07.007
American Diabetes Association (2008) Economic Costs of Diabetes in the U.S. in 2007. Diabetes Care 31:596–615. https://doi.org/10.2337/dc08-9017
Harrington C, Zagari MJ, Corea J, Klitenic J (2000) A cost analysis of diabetic lower-extremity ulcers. Diabetes Care 23:1333–1338. https://doi.org/10.2337/diacare.23.9.1333
Fowler MJ (2008) Microvascular and macrovascular complications of diabetes. Clinical Diabetes 26:77–82. https://doi.org/10.2337/diaclin.26.2.77
Oliver TI, Mutluoglu M (2022) Diabetic Foot Ulcer. StatPearls. StatPearls Publishing, Treasure Island (FL)
Volmer-Thole M, Lobmann R (2016) Neuropathy and diabetic foot syndrome. Int J Mol Sci 17(6):917. https://doi.org/10.3390/ijms17060917
Boulton AJM (2014) Diabetic neuropathy and foot complications. Handb Clin Neurol 126:97–107. https://doi.org/10.1016/B978-0-444-53480-4.00008-4
Bermudez DM, Herdrich BJ, Xu J et al (2011) Impaired biomechanical properties of diabetic skin: implications in pathogenesis of diabetic wound complications. Am J Pathol 178:2215–2223. https://doi.org/10.1016/j.ajpath.2011.01.015
Connizzo BK, Bhatt PR, Liechty KW, Soslowsky LJ (2014) Diabetes alters mechanical properties and collagen fiber re-alignment in multiple mouse tendons. Ann Biomed Eng 42:1880. https://doi.org/10.1007/s10439-014-1031-7
Berlanga-Acosta JA, Guillén-Nieto GE, Rodríguez-Rodríguez N et al (2020) Cellular senescence as the pathogenic hub of diabetes-related wound chronicity. Front Endocrinol 11:573032. https://doi.org/10.3389/fendo.2020.573032
Wilkinson HN, Hardman MJ (2021) Wound senescence: A functional link between diabetes and ageing? Exp Dermatol 30:68–73. https://doi.org/10.1111/exd.14082
de Macedo GMC, Nunes S, Barreto T (2016) Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr 8:63. https://doi.org/10.1186/s13098-016-0176-y
Xie X, Liu X, Li Y et al (2020) Advanced glycation end products enhance biofilm formation by promoting extracellular DNA release through sigb upregulation in Staphylococcus aureus. Front Microbiol. https://doi.org/10.3389/fmicb.2020.01479
Acosta JB, Garcia del Barco D, Cibrian Vera D et al (2008) The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J 5:530–539. https://doi.org/10.1111/j.1742-481X.2008.00457.x
Dinh T, Tecilazich F, Kafanas A et al (2012) Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 61:2937–2947. https://doi.org/10.2337/db12-0227
Frykberg RG, Banks J (2015) Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 4:560–582. https://doi.org/10.1089/wound.2015.0635
Rosner K, Ross C, Karlsmark T et al (1995) Immunohistochemical characterization of the cutaneous cellular infiltrate in different areas of chronic leg ulcers. APMIS 103:293–299. https://doi.org/10.1111/j.1699-0463.1995.tb01109.x
Loots MA, Lamme EN, Zeegelaar J et al (1998) Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 111:850–857. https://doi.org/10.1046/j.1523-1747.1998.00381.x
Sindrilaru A, Peters T, Wieschalka S et al (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121:985–997. https://doi.org/10.1172/JCI44490
Fadini GP, Menegazzo L, Rigato M et al (2016) NETosis delays diabetic wound healing in mice and humans. Diabetes 65:1061–1071. https://doi.org/10.2337/db15-0863
Wong SL, Demers M, Martinod K et al (2015) Diabetes primes neutrophils to undergo NETosis which severely impairs wound healing. Nat Med 21:815–819. https://doi.org/10.1038/nm.3887
Njeim R, Azar WS, Fares AH et al (2020) NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol 65:R65–R76. https://doi.org/10.1530/JME-20-0128
Wilgus TA, Roy S, McDaniel JC (2013) Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care 2:379–388. https://doi.org/10.1089/wound.2012.0383
Bannon P, Wood S, Restivo T et al (2013) Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech 6:1434–1447. https://doi.org/10.1242/dmm.012237
Hesketh M, Sahin KB, West ZE, Murray RZ (2017) Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci 18(7):1545. https://doi.org/10.3390/ijms18071545
Khanna S, Biswas S, Shang Y et al (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 5:e9539. https://doi.org/10.1371/journal.pone.0009539
Yager DR, Nwomeh BC (1999) The proteolytic environment of chronic wounds. Wound Repair Regen 7:433–441. https://doi.org/10.1046/j.1524-475x.1999.00433.x
Mast BA, Schultz GS (1996) Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Rep Reg 4:411–420. https://doi.org/10.1046/j.1524-475X.1996.40404.x
Bauer SM, Bauer RJ, Velazquez OC (2005) Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovasc Surg 39:293–306. https://doi.org/10.1177/153857440503900401
Zhou K, Ma Y, Brogan MS (2015) Chronic and non-healing wounds: The story of vascular endothelial growth factor. Med Hypotheses 85:399–404. https://doi.org/10.1016/j.mehy.2015.06.017
Fadini GP, Sartore S, Agostini C, Avogaro A (2007) Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 30:1305–1313. https://doi.org/10.2337/dc06-2305
Kim K-A, Shin Y-J, Kim J-H et al (2012) Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm Res 35:223–234. https://doi.org/10.1007/s12272-012-0203-y
Westerweel PE, Teraa M, Rafii S et al (2013) Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS ONE 8(3):e60357. https://doi.org/10.1371/journal.pone.0060357
Usui ML, Mansbridge JN, Carter WG et al (2008) Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem 56:687–696. https://doi.org/10.1369/jhc.2008.951194
Stojadinovic O, Brem H, Vouthounis C et al (2005) Molecular pathogenesis of chronic wounds: the role of β-Catenin and c-myc in the Inhibition of epithelialization and wound healing. Am J Pathol 167:59–69. https://doi.org/10.1016/S0002-9440(10)62953-7
Galkowska H, Olszewsk WL, Wojewodzka U et al (2003) Expression of apoptosis- and cell cycle-related proteins in epidermis of venous leg and diabetic foot ulcers. Surgery 134:213–220. https://doi.org/10.1067/msy.2003.223
Wan R, Weissman JP, Grundman K et al (2021) Diabetic wound healing: The impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Rep Reg 29:573–581. https://doi.org/10.1111/wrr.12954
Cook H, Stephens P, Davies KJ et al (2000) Defective Extracellular Matrix Reorganization by Chronic Wound Fibroblasts is Associated with Alterations in TIMP-1, TIMP-2, and MMP-2 Activity. J Investig Dermatol 115:225–233. https://doi.org/10.1046/j.1523-1747.2000.00044.x
Levinson H (2013) A paradigm of fibroblast activation and dermal wound contraction to guide the development of therapies for chronic wounds and pathologic scars. Adv Wound Care 2:149–159. https://doi.org/10.1089/wound.2012.0389
Blakytny R, Jude E (2006) The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 23:594–608. https://doi.org/10.1111/j.1464-5491.2006.01773.x
Ochoa O, Torres FM, Shireman PK (2007) Chemokines and diabetic wound healing. Vascular 15:350–355. https://doi.org/10.2310/6670.2007.00056
Wetzler C, Kämpfer H, Stallmeyer B et al (2000) Large and sustained induction of Chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Investig Dermatol 115:245–253. https://doi.org/10.1046/j.1523-1747.2000.00029.x
Wood S, Jayaraman V, Huelsmann EJ et al (2014) Pro-inflammatory chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response. PLoS ONE 9:e91574. https://doi.org/10.1371/journal.pone.0091574
Fivenson DP, Faria DT, Nickoloff BJ et al (1997) Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Rep Regen 5:310–322. https://doi.org/10.1046/j.1524-475X.1997.50405.x
Gallagher KA, Liu Z-J, Xiao M et al (2007) Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J Clin Invest 117:1249–1259. https://doi.org/10.1172/JCI29710
Bekeschus S, Schmidt A, Napp M et al (2017) Distinct cytokine and chemokine patterns in chronic diabetic ulcers and acute wounds. Exp Dermatol 26:145–147. https://doi.org/10.1111/exd.13215
Zhang Y, Thai K, Kepecs DM et al (2018) Reversing CXCL10 deficiency ameliorates kidney disease in diabetic mice. Am J Pathol 188:2763–2773. https://doi.org/10.1016/j.ajpath.2018.08.017
Barrientos S, Stojadinovic O, Golinko MS et al (2008) PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Rep Reg 16:585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x
Trengove NJ, Bielefeldt-Ohmann H, Stacey MC (2000) Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Rep Regen 8:13–25. https://doi.org/10.1046/j.1524-475x.2000.00013.x
Wen Y, Gu J, Li S-L et al (2006) Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 147:2518–2525. https://doi.org/10.1210/en.2005-0519
Bitar MS, Labbad ZN (1996) Transforming growth factor-β and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 61:113–119. https://doi.org/10.1006/jsre.1996.0090
Okizaki S, Ito Y, Hosono K et al (2015) Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmacother 70:317–325. https://doi.org/10.1016/j.biopha.2014.10.020
Doxey DL, Ng MC, Dill RE, Iacopino AM (1995) Platelet-derived growth factor levels in wounds of diabetic rats. Life Sci 57:1111–1123. https://doi.org/10.1016/0024-3205(95)02056-o
Robson MC (1997) The role of growth factors in the healing of chronic wounds. Wound Rep Regen 5:12–17. https://doi.org/10.1046/j.1524-475X.1997.50106.x
Ramirez H, Patel SB, Pastar I (2014) The role of TGFβ signaling in wound epithelialization. Adv Wound Care 3:482. https://doi.org/10.1089/wound.2013.0466
Maione AG, Smith A, Kashpur O et al (2016) Altered ECM Deposition by Diabetic Foot Ulcer-Derived Fibroblasts Implicates Fibronectin in Chronic Wound Repair. Wound Rep Reg Off Pub Wound Heal Soc European Tissue Rep Soc 24:630. https://doi.org/10.1111/wrr.12437
Zubair M, Ahmad J (2019) Role of growth factors and cytokines in diabetic foot ulcer healing: a detailed review. Rev Endocr Metab Disord 20:207–217. https://doi.org/10.1007/s11154-019-09492-1
Suvas S (2017) Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol 199:1543–1552. https://doi.org/10.4049/jimmunol.1601751
Ekstrand AJ, Cao R, Bjorndahl M et al (2003) Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA 100:6033–6038. https://doi.org/10.1073/pnas.1135965100
Toda M, Suzuki T, Hosono K et al (2008) Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother 62:352–359. https://doi.org/10.1016/j.biopha.2008.02.003
Pradhan L, Nabzdyk C, Andersen ND et al (2009) Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med 11:e2. https://doi.org/10.1017/S1462399409000945
Lobmann R, Ambrosch A, Schultz G et al (2002) Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 45:1011–1016. https://doi.org/10.1007/s00125-002-0868-8
Subramaniam K, Pech CM, Stacey MC, Wallace HJ (2008) Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid*. Int Wound J 5:79–86. https://doi.org/10.1111/j.1742-481X.2007.00336.x
Wysocki AB, Staiano-Coico L, Grinnell F (1993) Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 101:64–68. https://doi.org/10.1111/1523-1747.ep12359590
Yang S, Gu Z, Lu C et al (2020) Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv Wound Care (New Rochelle) 9:16–27. https://doi.org/10.1089/wound.2019.0943
Arpino V, Brock M, Gill SE (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44–46:247–254. https://doi.org/10.1016/j.matbio.2015.03.005
Vaalamo M, Leivo T, Saarialho-Kere U (1999) Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, – 2, – 3, and – 4) in normal and aberrant wound healing. Hum Pathol 30:795–802. https://doi.org/10.1016/s0046-8177(99)90140-5
Vaalamo M, Weckroth M, Puolakkainen P et al (1996) Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 135:52–59
Negre-Salvayre A, Salvayre R, Augé N et al (2009) Hyperglycemia and glycation in diabetic complications. Antioxid Redox Sig 11:3071–3109. https://doi.org/10.1089/ars.2009.2484
Ramasamy R, Yan SF, Schmidt AM (2011) Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann NY Acad Sci 1243:88–102. https://doi.org/10.1111/j.1749-6632.2011.06320.x
Schreml S, Szeimies RM, Prantl L et al (2010) Oxygen in acute and chronic wound healing. Br J Dermatol 163:257–268. https://doi.org/10.1111/j.1365-2133.2010.09804.x
Weiss SJ (1989) Tissue destruction by neutrophils. N Engl J Med 320:365–376. https://doi.org/10.1056/NEJM198902093200606
Kunkemoeller B, Kyriakides TR (2017) Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Sig 27:823. https://doi.org/10.1089/ars.2017.7263
Wicks K, Torbica T, Mace KA (2014) Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin Immunol 26:341–353. https://doi.org/10.1016/j.smim.2014.04.006
Barman PK, Urao N, Koh TJ (2019) Diabetes induces myeloid bias in bone marrow progenitors associated with enhanced wound macrophage accumulation and impaired healing. J Pathol 249:435–446. https://doi.org/10.1002/path.5330
Joshi N, Pohlmeier L, Ben-Yehuda Greenwald M et al (2020) Comprehensive characterization of myeloid cells during wound healing in healthy and healing-impaired diabetic mice. Eur J Immunol 50:1335–1349. https://doi.org/10.1002/eji.201948438
Kimball A, Schaller M, Joshi A et al (2018) Ly6CHi blood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler Thromb Vasc Biol 38:1102–1114. https://doi.org/10.1161/ATVBAHA.118.310703
Mahdipour E, Charnock JC, Mace KA (2011) Hoxa3 promotes the differentiation of hematopoietic progenitor cells into proangiogenic Gr-1+CD11b+ myeloid cells. Blood 117:815–826. https://doi.org/10.1182/blood-2009-12-259549
Gallagher KA, Joshi A, Carson WF et al (2014) Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 64:1420–1430. https://doi.org/10.2337/db14-0872
Tian M, Qing C, Niu Y et al (2013) Effect of aminoguanidine intervention on neutrophils in diabetes inflammatory cells wound healing. Exp Clin Endocrinol Diabetes 121:635–642. https://doi.org/10.1055/s-0033-1351331
Tian M, Qing C, Niu Y et al (2016) The relationship between inflammation and impaired wound healing in a diabetic rat burn model. J Burn Care Res 37:e115–e124. https://doi.org/10.1097/BCR.0000000000000171
Diegelmann RF (2003) Excessive neutrophils characterize chronic pressure ulcers. Wound Rep Reg 11:490–495. https://doi.org/10.1046/j.1524-475X.2003.11617.x
McDaniel JC, Roy S, Wilgus TA (2013) Neutrophil activity in chronic venous leg ulcers—A target for therapy? Wound Rep Reg Off Pub Wound Heal Soc European Tissue Rep Soc 21:339. https://doi.org/10.1111/wrr.12036
Wlaschek M, Scharffetter-Kochanek K (2005) Oxidative stress in chronic venous leg ulcers. Wound Rep Regen 13:452–461. https://doi.org/10.1111/j.1067-1927.2005.00065.x
Sawaya AP, Stone RC, Brooks SR et al (2020) Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun 11:4678. https://doi.org/10.1038/s41467-020-18276-0
Theocharidis G, Baltzis D, Roustit M et al (2020) Integrated Skin transcriptomics and serum multiplex assays reveal novel mechanisms of wound healing in diabetic foot ulcers. Diabetes 69:2157–2169. https://doi.org/10.2337/db20-0188
Mirza RE, Fang MM, Ennis WJ, Koh TJ (2013) Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 Diabetes. Diabetes 62:2579–2587. https://doi.org/10.2337/db12-1450
Mirza RE, Fang MM, Novak ML et al (2015) Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol 236:433–444. https://doi.org/10.1002/path.4548
Mirza RE, Fang MM, Weinheimer-Haus EM et al (2014) Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 63:1103–1114. https://doi.org/10.2337/db13-0927
Yager DR, Chen SM, Ward SI et al (1997) Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Rep Reg 5:23–32. https://doi.org/10.1046/j.1524-475X.1997.50108.x
Rao CN, Ladin DA, Liu YY et al (1995) Alpha 1-antitrypsin is degraded and non-functional in chronic wounds but intact and functional in acute wounds: the inhibitor protects fibronectin from degradation by chronic wound fluid enzymes. J Invest Dermatol 105:572–578. https://doi.org/10.1111/1523-1747.ep12323503
Grinnell F, Zhu M (1996) Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol 106:335–341. https://doi.org/10.1111/1523-1747.ep12342990
Fadini GP, Albiero M, Millioni R et al (2014) The molecular signature of impaired diabetic wound healing identifies serpinB3 as a healing biomarker. Diabetologia 57:1947–1956. https://doi.org/10.1007/s00125-014-3300-2
Wang Y, Shao T, Wang J et al (2021) An update on potential biomarkers for diagnosing diabetic foot ulcer at early stage. Biomed Pharmacother 133:110991. https://doi.org/10.1016/j.biopha.2020.110991
Raghavan JV, Sagar SK, Dorai VK et al (2022) Cholesterol levels and monocyte phenotype are predictors of nonhealing in individuals with low-grade diabetic foot ulcers: a prospective cohort study. Adv Wound Care (New Rochelle). https://doi.org/10.1089/wound.2021.0182
Cole JB, Florez JC (2020) Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 16:377–390. https://doi.org/10.1038/s41581-020-0278-5
Viswanathan V, Dhamodharan U, Srinivasan V et al (2018) Single nucleotide polymorphisms in cytokine/chemokine genes are associated with severe infection, ulcer grade and amputation in diabetic foot ulcer. Int J Biol Macromol 118:1995–2000. https://doi.org/10.1016/j.ijbiomac.2018.07.083
Zhao J, Zhang L-X, Wang Y-T et al (2022) Genetic polymorphisms and the risk of diabetic foot: a systematic review and meta-analyses. Int J Low Extrem Wounds 21:574–587. https://doi.org/10.1177/1534734620977599
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867. https://doi.org/10.1038/nature05485
Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:98–107. https://doi.org/10.1038/nri2925
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116:1793–1801. https://doi.org/10.1172/JCI29069
Dandona P, Weinstock R, Thusu K et al (1998) Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab 83:2907–2910. https://doi.org/10.1210/jcem.83.8.5026
Hotamisligil GS, Arner P, Caro JF et al (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409–2415. https://doi.org/10.1172/JCI117936
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91. https://doi.org/10.1126/science.7678183
Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA (2009) Islet Inflammation Impairs the Pancreatic β-Cell in Type 2 Diabetes. Physiology 24:325–331. https://doi.org/10.1152/physiol.00032.2009
Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116:1802–1812. https://doi.org/10.1172/JCI29103
Sun T, Han X (2020) Death versus dedifferentiation: The molecular bases of beta cell mass reduction in type 2 diabetes. Semin Cell Dev Biol 103:76–82. https://doi.org/10.1016/j.semcdb.2019.12.002
Weir GC, Bonner-Weir S (2004) Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53:S16–S21. https://doi.org/10.2337/diabetes.53.suppl_3.S16
Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R (2020) Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev 16:442–449. https://doi.org/10.2174/1573399815666191024085838
Kahn BB (1998) Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92:593–596. https://doi.org/10.1016/S0092-8674(00)81125-3
Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R (2011) State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun 12:239. https://doi.org/10.1038/gene.2011.14
Herder C, Brunner EJ, Rathmann W et al (2009) Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the whitehall II study. Diabetes Care 32:421. https://doi.org/10.2337/dc08-1161
Pradhan AD, Manson JE, Rifai N et al (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334. https://doi.org/10.1001/jama.286.3.327
Spranger J, Kroke A, Möhlig M et al (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52:812–817. https://doi.org/10.2337/diabetes.52.3.812
Pickup JC, Mattock MB, Chusney GD, Burt D (1997) NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40:1286–1292. https://doi.org/10.1007/s001250050822
Vasamsetti SB, Florentin J, Coppin E et al (2018) Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity 49:93-106.e7. https://doi.org/10.1016/j.immuni.2018.05.004
Zhang H, Yang Z, Zhang W et al (2017) White blood cell subtypes and risk of type 2 diabetes. J Diabetes Complicat 31:31–37. https://doi.org/10.1016/j.jdiacomp.2016.10.029
Carestia A, Frechtel G, Cerrone G et al (2016) NETosis before and after Hyperglycemic Control in Type 2 Diabetes Mellitus Patients. PLoS ONE 11:e0168647. https://doi.org/10.1371/journal.pone.0168647
García AG, Rodríguez MR, Alonso CG et al (2015) Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab J 39:59. https://doi.org/10.4093/dmj.2015.39.1.59
Menegazzo L, Ciciliot S, Poncina N et al (2015) NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 52:497–503. https://doi.org/10.1007/s00592-014-0676-x
Alba-Loureiro TC, Munhoz CD, Martins JO et al (2007) Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res 40:1037–1044. https://doi.org/10.1590/s0100-879x2006005000143
Dasu MR, Devaraj S, Jialal I (2007) High glucose induces IL-1β expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab 293:E337–E346. https://doi.org/10.1152/ajpendo.00718.2006
Giulietti A, Stoffels K, Decallonne B et al (2004) Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann NY Acad Sci 1037:74–78. https://doi.org/10.1196/annals.1337.011
Ratter JM, van Heck JIP, Rooijackers HMM et al (2021) Insulin acutely activates metabolism of primary human monocytes and promotes a proinflammatory phenotype. J Leukoc Biol 110:885–891. https://doi.org/10.1002/JLB.3AB0120-019RR
Shanmugam N, Reddy MA, Guha M, Natarajan R (2003) High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52:1256–1264. https://doi.org/10.2337/diabetes.52.5.1256
Raghavan JV, Dorai VK, Sagar SK et al (2022) Immunomodulatory bandage for accelerated healing of diabetic wounds. ACS Bio Med Chem Au 2:409–418. https://doi.org/10.1021/acsbiomedchemau.1c00063
Everett E, Mathioudakis N (2018) Update on management of diabetic foot ulcers. Ann NY Acad Sci 1411:153–165. https://doi.org/10.1111/nyas.13569
Armstrong DG, Lipsky BA (2004) Diabetic foot infections: stepwise medical and surgical management. Int Wound J 1:123–132. https://doi.org/10.1111/j.1742-4801.2004.00035.x
Frykberg RG, Wukich DK, Kavarthapu V et al (2020) Surgery for the diabetic foot: A key component of care. Diabetes/Metab Res Rev 36:e3251. https://doi.org/10.1002/dmrr.3251
Larsson J, Agardh C-D, Apelqvist J, Stenström A (1998) Long term prognosis after healed amputation in patients with diabetes. Clin Orthop Relat Res 350:149
Armstrong DG, Lavery LA, Diabetic Foot Study Consortium (2005) Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 366:1704–1710. https://doi.org/10.1016/S0140-6736(05)67695-7
Blume PA, Walters J, Payne W et al (2008) Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 31:631–636. https://doi.org/10.2337/dc07-2196
Hasan MY, Teo R, Nather A (2015) Negative-pressure wound therapy for management of diabetic foot wounds: a review of the mechanism of action, clinical applications, and recent developments. Diabetic Foot Ankle 6:27618. https://doi.org/10.3402/dfa.v6.27618
Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H (2008) A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 95:685–692. https://doi.org/10.1002/bjs.6238
Abidia A, Laden G, Kuhan G et al (2003) The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg 25:513–518. https://doi.org/10.1053/ejvs.2002.1911
Roeckl-Wiedmann I, Bennett M, Kranke P (2005) Systematic review of hyperbaric oxygen in the management of chronic wounds. Br J Surg 92:24–32. https://doi.org/10.1002/bjs.4863
Stoekenbroek RM, Santema TB, Legemate DA et al (2014) Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg 47:647–655. https://doi.org/10.1016/j.ejvs.2014.03.005
Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG et al (2019) Current therapeutic strategies in diabetic foot ulcers. Medicina (Kaunas) 55:714. https://doi.org/10.3390/medicina55110714
Pourmoussa A, Gardner DJ, Johnson MB, Wong AK (2016) An update and review of cell-based wound dressings and their integration into clinical practice. Ann Trans Med 4(23):457. https://doi.org/10.2103/atm.2016.12.44
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound healing: A cellular perspective. Physiol Rev 99:665–706. https://doi.org/10.1152/physrev.00067.2017
Mostow EN, Haraway GD, Dalsing M et al (2005) Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J Vasc Surg 41:837–843. https://doi.org/10.1016/j.jvs.2005.01.042
Niezgoda JA, Van Gils CC, Frykberg RG et al (2005) Randomized clinical trial comparing OASIS wound matrix to regranex gel for diabetic ulcers. Adv Skin Wound Care 18:258–266
Driver VR, Lavery LA, Reyzelman AM et al (2015) A clinical trial of integra template for diabetic foot ulcer treatment. Wound Rep Regen 23:891–900. https://doi.org/10.1111/wrr.12357
Zelen CM, Gould L, Serena TE et al (2015) A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 12:724–732. https://doi.org/10.1111/iwj.12395
Zelen CM, Serena TE, Denoziere G, Fetterolf DE (2013) A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 10:502–507. https://doi.org/10.1111/iwj.12097
Cazzell S, Vayser D, Pham H et al (2017) A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Rep Regen 25:483–497. https://doi.org/10.1111/wrr.12551
Guo X, Mu D, Gao F (2017) Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: A systematic review and meta-analysis. Int J Surg 40:1–7. https://doi.org/10.1016/j.ijsu.2017.02.008
Falanga V, Sabolinski M (1999) A bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Rep Reg 7:201–207. https://doi.org/10.1046/j.1524-475X.1999.00201.x
Zaulyanov L, Kirsner RS (2007) A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2:93–98. https://doi.org/10.2147/ciia.2007.2.1.93
Hart CE, Loewen-Rodriguez A, Lessem J (2012) Dermagraft: use in the treatment of chronic wounds. Adv Wound Care 1:138–141. https://doi.org/10.1089/wound.2011.0282
Marston WA, Hanft J, Norwood P et al (2003) The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26:1701–1705. https://doi.org/10.2337/diacare.26.6.1701
Nicholls H (2001) FDA approves Dermagraft® for diabetic foot ulcers. Trends Endocrinol Metab 12:433. https://doi.org/10.1016/S1043-2760(01)00536-7
Gibbons GW (2015) Grafix®, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv Wound Care 4:534–544. https://doi.org/10.1089/wound.2015.0647
Lavery LA, Fulmer J, Shebetka KA et al (2014) The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 11:554–560. https://doi.org/10.1111/iwj.12329
Vericel Research Group C for BE and (2019) Epicel (cultured epidermal autografts). FDA
Carsin H, Ainaud P, Le Bever H et al (2000) Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns 26:379–387. https://doi.org/10.1016/S0305-4179(99)00143-6
Patel S, Srivastava S, Singh MR, Singh D (2019) Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother 112:108615. https://doi.org/10.1016/j.biopha.2019.108615
Smiell JM, Wieman TJ, Steed DL et al (1999) Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Rep Regen 7:335–346. https://doi.org/10.1046/j.1524-475x.1999.00335.x
Rayman G, Vas P, Dhatariya K et al (2020) Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes/Metab Res Rev 36:e3283. https://doi.org/10.1002/dmrr.3283
Vas P, Rayman G, Dhatariya K et al (2020) Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: a systematic review. Diabetes/Metab Res Rev 36:e3284. https://doi.org/10.1002/dmrr.3284
Game FL, Apelqvist J, Attinger C et al (2016) Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev 32(Suppl 1):154–168. https://doi.org/10.1002/dmrr.2707
Game FL, Hinchliffe RJ, Apelqvist J et al (2012) A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 28(Suppl 1):119–141. https://doi.org/10.1002/dmrr.2246
Tyler SEB (2017) Nature’s electric potential: a systematic review of the role of bioelectricity in wound healing and regenerative processes in animals, humans, and plants. Front Physiol 8:627. https://doi.org/10.3389/fphys.2017.00627
Mirpour S, Fathollah S, Mansouri P et al (2020) Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci Rep 10:10440. https://doi.org/10.1038/s41598-020-67232-x
Stratmann B, Costea TC, Nolte C et al (2020) Effect of cold atmospheric plasma therapy vs standard therapy placebo on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw Open 3:e2010411. https://doi.org/10.1001/jamanetworkopen.2020.10411
Edmonds M, Lázaro-Martínez JL, Alfayate-García JM et al (2018) Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol 6:186–196. https://doi.org/10.1016/S2213-8587(17)30438-2
Snyder D, Sullivan N, Margolis D, Schoelles K (2020) Skin Substitutes for Treating Chronic Wounds. Agency for Healthcare Research and Quality (US), Rockville (MD)
Game F, Jeffcoate W, Tarnow L et al (2018) LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol 6:870–878. https://doi.org/10.1016/S2213-8587(18)30240-7
Tettelbach W, Cazzell S, Sigal F et al (2019) A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int Wound J 16:122–130. https://doi.org/10.1111/iwj.13001
Ananian CE, Dhillon YS, Van Gils CC et al (2018) A multicenter, randomized, single-blind trial comparing the efficacy of viable cryopreserved placental membrane to human fibroblast-derived dermal substitute for the treatment of chronic diabetic foot ulcers. Wound Rep Regen 26:274–283. https://doi.org/10.1111/wrr.12645
DiDomenico LA, Orgill DP, Galiano RD et al (2016) Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open 4:e1095. https://doi.org/10.1097/GOX.0000000000001095
Huang Y-Z, Gou M, Da L-C et al (2020) Mesenchymal stem cells for chronic wound healing: current status of preclinical and Clinical Studies. Tissue Eng Part B Rev 26:555–570. https://doi.org/10.1089/ten.TEB.2019.0351
Öhnstedt E, Lofton Tomenius H, Vågesjö E, Phillipson M (2019) The discovery and development of topical medicines for wound healing. Expert Opin Drug Discov 14:485–497. https://doi.org/10.1080/17460441.2019.1588879
Mohammadi MH, Molavi B, Mohammadi S et al (2017) Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Trans Apher Sci 56:160–164. https://doi.org/10.1016/j.transci.2016.10.020
Qu S, Hu Z, Zhang Y et al (2022) Clinical studies on platelet-rich plasma therapy for chronic cutaneous ulcers: a systematic review and meta-analysis of randomized controlled trials. Adv Wound Care 11:56–69. https://doi.org/10.1089/wound.2020.1186
Akbarzadeh S, McKenzie MB, Rahman MM, Cleland H (2021) Allogeneic platelet-rich plasma: is it safe and effective for wound repair? ESR 62:1–9. https://doi.org/10.1159/000514223
Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I et al (2016) Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006899.pub3
Suthar M, Gupta S, Bukhari S, Ponemone V (2017) Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci 24:16. https://doi.org/10.1186/s12929-017-0324-1
Huang YY, Lin CW, Cheng NC et al (2021) Effect of a novel macrophage-regulating drug on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw Open 4:e2122607. https://doi.org/10.1001/jamanetworkopen.2021.22607
Masson-Meyers DS, Andrade TAM, Caetano GF et al (2020) Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol 101:21–37. https://doi.org/10.1111/iep.12346
Parnell LKS, Volk SW (2019) The evolution of animal models in wound healing research: 1993–2017. Adv Wound Care 8:692. https://doi.org/10.1089/wound.2019.1098
Kwan RL-C, Lu S, Choi HM-C et al (2019) Efficacy of biophysical energies on healing of diabetic skin wounds in cell studies and animal experimental models: a systematic review. Int J Mol Sci 20:368. https://doi.org/10.3390/ijms20020368
Callaghan MJ, Chang EI, Seiser N et al (2008) Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF-2 release. Plast Reconstr Surg 121:130–141. https://doi.org/10.1097/01.prs.0000293761.27219.84
Choi HMC, Cheing AKK, Ng GYF, Cheing GLY (2018) Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing. PLoS ONE 13:e0191074. https://doi.org/10.1371/journal.pone.0191074
Hayashi D, Kawakami K, Ito K et al (2012) Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: a critical role of endothelial nitric oxide synthase. Wound Rep Regen 20:887–895. https://doi.org/10.1111/j.1524-475X.2012.00851.x
Kuo Y-R, Wang C-T, Wang F-S et al (2009) Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Rep Regen 17:522–530. https://doi.org/10.1111/j.1524-475X.2009.00504.x
de Alencar J, Santos F, Campelo MBD, Alencar R, de Oliveira et al (2018) Effects of low-power light therapy on the tissue repair process of chronic wounds in diabetic feet. Photomed Laser Surg 36:298–304. https://doi.org/10.1089/pho.2018.4455
Mostafavinia A, Amini A, Ahmadi H et al (2021) Combined treatment of Photobiomodulation and arginine on chronic wound healing in an animal model. J Lasers Med Sci. https://doi.org/10.34172/jlms.2021.40
Maharlooei MK, Bagheri M, Solhjou Z et al (2011) Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract 93:228–234. https://doi.org/10.1016/j.diabres.2011.04.018
Kim SW, Zhang HZ, Guo L et al (2012) Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS ONE 7:e41105. https://doi.org/10.1371/journal.pone.0041105
Ma D, Kua JEH, Lim WK et al (2015) In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy 17:1036–1051. https://doi.org/10.1016/j.jcyt.2015.04.001
Kuo YR, Wang CT, Cheng JT et al (2011) Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of Streptozotocin-induced diabetes. Plast Reconstr Surg 128:872–880. https://doi.org/10.1097/PRS.0b013e3182174329
Sierra-Sánchez Á, Kim KH, Blasco-Morente G, Arias-Santiago S (2021) Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. npj Regen Med 6:1–23. https://doi.org/10.1038/s41536-021-00144-0
Lee K-B, Choi J, Cho S-B et al (2011) Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res 29:1554–1562. https://doi.org/10.1002/jor.21385
Pedroso DCS, Tellechea A, Moura L et al (2011) Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial Cells. PLoS ONE 6:e16114. https://doi.org/10.1371/journal.pone.0016114
Irons RF, Cahill KW, Rattigan DA et al (2018) Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J Vasc Surg 68:115S-125S. https://doi.org/10.1016/j.jvs.2018.01.065
Kazemi-Darabadi S, Sarrafzadeh-Rezaei F, Farshid A-A, Dalir-Naghadeh B (2014) Allogenous skin fibroblast transplantation enhances excisional wound healing following alloxan diabetes in sheep, a randomized controlled trial. Int J Surg 12:751–756. https://doi.org/10.1016/j.ijsu.2014.06.007
Velander P, Theopold C, Bleiziffer O et al (2009) Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res 157:14–20. https://doi.org/10.1016/j.jss.2008.10.001
Brown RL, Breeden MP, Greenhalgh DG (1994) PDGF and TGF-α act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res 56:562–570. https://doi.org/10.1006/jsre.1994.1090
Chan RK, Liu PH, Pietramaggiori G et al (2006) Effect of recombinant platelet-derived growth factor (Regranex®) on wound closure in genetically diabetic mice. J Burn Care Res 27:202–205. https://doi.org/10.1097/01.BCR.0000202898.11277.58
Cheng B, Liu H-W, Fu X-B et al (2007) Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci 45:193–201. https://doi.org/10.1016/j.jdermsci.2006.11.014
Judith R, Nithya M, Rose C, Mandal AB (2010) Application of a PDGF-containing novel gel for cutaneous wound healing. Life Sci 87:1–8. https://doi.org/10.1016/j.lfs.2010.05.003
Mizuno K, Yamamura K, Yano K et al (2003) Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res Part A 64:177–181. https://doi.org/10.1002/jbm.a.10396
Wang W, Lin S, Xiao Y et al (2008) Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci 82:190–204. https://doi.org/10.1016/j.lfs.2007.11.009
Yang Y, Xia T, Zhi W et al (2011) Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 32:4243–4254. https://doi.org/10.1016/j.biomaterials.2011.02.042
Choi JS, Leong KW, Yoo HS (2008) In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 29:587–596. https://doi.org/10.1016/j.biomaterials.2007.10.012
Dong X, Xu J, Wang W et al (2008) Repair effect of diabetic ulcers with recombinant human epidermal growth factor loaded by sustained-release microspheres. SCI China Ser C 51:1039–1044. https://doi.org/10.1007/s11427-008-0126-5
Hardwicke JT, Hart J, Bell A et al (2011) The effect of dextrin-rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J Control Release 152:411–417. https://doi.org/10.1016/j.jconrel.2011.03.016
Matsumoto Y, Kuroyanagi Y (2010) Development of a wound dressing composed of hyaluronic acid sponge containing arginine and epidermal growth factor. J Biomater Sci Polym Ed 21:715–726. https://doi.org/10.1163/156856209X435844
Pyun DG, Choi HJ, Yoon HS et al (2015) Polyurethane foam containing rhEGF as a dressing material for healing diabetic wounds: Synthesis, characterization, in vitro and in vivo studies. Colloids Surf B 135:699–706. https://doi.org/10.1016/j.colsurfb.2015.08.029
Tsang MW, Wong WKR, Hung CS et al (2003) Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 26:1856–1861. https://doi.org/10.2337/diacare.26.6.1856
Chereddy KK, Lopes A, Koussoroplis S et al (2015) Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. nanomedicine: nanotechnology. Biol Med 11:1975–1984. https://doi.org/10.1016/j.nano.2015.07.006
Xie Z, Paras CB, Weng H et al (2013) Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 9:9351–9359. https://doi.org/10.1016/j.actbio.2013.07.030
Yan X, Chen B, Lin Y et al (2010) Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract 90:66–72. https://doi.org/10.1016/j.diabres.2010.07.001
Koria P, Yagi H, Kitagawa Y et al (2011) Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. PNAS 108:1034–1039. https://doi.org/10.1073/pnas.1009881108
Miller TJ, Henson K, Aras R et al (2013) Stromal cell-derived factor-1 non-viral DNA therapy accelerates wound healing and decreases scar formation in porcine surgical incisions. Mol Ther 21:S193. https://doi.org/10.1016/S1525-0016(16)34835-3
Vågesjö E, Öhnstedt E, Mortier A et al (2018) Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci USA 115:1895–1900. https://doi.org/10.1073/pnas.1716580115
Kaur T, Dumoga S, Koul V, Singh N (2020) Modulating neutrophil extracellular traps for wound healing. Biomater Sci 8:3212–3223. https://doi.org/10.1039/D0BM00355G
Cam ME, Ertas B, Alenezi H et al (2021) Accelerated diabetic wound healing by topical application of combination oral antidiabetic agents-loaded nanofibrous scaffolds: An in vitro and in vivo evaluation study. Mater Sci Eng C Mater Biol Appl 119:111586. https://doi.org/10.1016/j.msec.2020.111586
Hamed S, Ullmann Y, Masoud M et al (2010) Topical Erythropoietin Promotes Wound Repair in Diabetic Rats. J Investig Dermatol 130:287–294. https://doi.org/10.1038/jid.2009.219
Kawai K, Kageyama A, Tsumano T et al (2008) Effects of adiponectin on growth and differentiation of human keratinocytes-Implication of impaired wound healing in diabetes. Biochem Biophys Res Commun 374:269–273. https://doi.org/10.1016/j.bbrc.2008.07.045
Leal EC, Carvalho E, Tellechea A et al (2015) Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol 185:1638–1648. https://doi.org/10.1016/j.ajpath.2015.02.011
Liu J, Yan L, Yang W et al (2019) Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact Mat 4:151–159. https://doi.org/10.1016/j.bioactmat.2019.03.001
Mouritzen MV, Abourayale S, Ejaz R et al (2018) Neurotensin, substance P, and insulin enhance cell migration. J Pept Sci 24:e3093. https://doi.org/10.1002/psc.3093
Wang J, Xu J (2020) Effects of topical insulin on wound healing: a review of animal and human evidences. Diabetes Metab Syndr Obes Targets Ther 13:719. https://doi.org/10.2147/DMSO.S237294
Babaei S, Bayat M, Nouruzian M, Bayat M (2013) Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol 700:165–172. https://doi.org/10.1016/j.ejphar.2012.11.024
Gooyit M, Peng Z, Wolter WR et al (2014) A chemical biological strategy to facilitate diabetic wound healing. ACS Chem Biol 9:105–110. https://doi.org/10.1021/cb4005468
Chong HC, Chan JSK, Goh CQ et al (2014) Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol Ther 22:1593–1604. https://doi.org/10.1038/mt.2014.102
Gallant-Behm CL, Piper J, Dickinson BA et al (2018) A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Rep Reg 26:311–323. https://doi.org/10.1111/wrr.12660
Gomes A, Teixeira C, Ferraz R et al (2017) Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules 22:1743. https://doi.org/10.3390/molecules22101743
Kasiewicz LN, Whitehead KA (2017) Recent advances in biomaterials for the treatment of diabetic foot ulcers. Biomater Sci 5:1962–1975. https://doi.org/10.1039/C7BM00264E
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raghavan, J.V., Jhunjhunwala, S. Role of Innate Immune Cells in Chronic Diabetic Wounds. J Indian Inst Sci 103, 249–271 (2023). https://doi.org/10.1007/s41745-022-00355-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-022-00355-4