Abstract
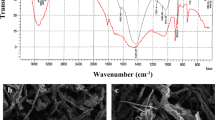
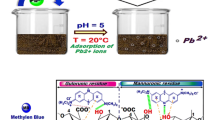
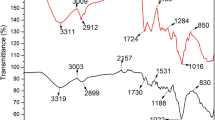
For eradication of Cu(II) and Pb(II), the biosorption performance of two biosorbents, which are single class Chlorophyceae alga and a mixture formed from Chlorophyceae; Cyanophyceae, and Bacillariophyceae algae, is being evaluated in the present work. For this purpose, the biosorption experiments were carried out on biomass samples of these algal classes and found to be sensitive towards variation in environmental conditions. Further, the characterization study performed using FT-IR and scanning electron micrograph techniques, revealed the presence of highly essential active groups, in addition to, several morphological properties on the surface of both biosorbents. The removal efficiency was found to change substantially, depending on the type of biosorbed metal, rather than algal biomass type. Additionally, the equilibrium isotherm study demonstrated that the biosorption mechanisms of the two metals differed for CA and MA, as well as, Langmuir and Temkin equations best fitted the isotherm data. Moreover, the kinetic data of Cu(II) and Pb(II) fitted well with Hill and Weibull-Avrami fractal models, respectively, illustrating that the kinetics of biosorption mechanism of Cu(II) onto one biosorbent differs from Pb(II). Upon comparing the obtained biosorption results, one can deduce that in the advanced treatment units of wastewater, MA acts as a more favorable biosorbent agent than CA. With the aim of further protecting the environment by reducing the percentage of solid waste generated from such treatment, the present study suggested the mixing of used algal biomass with cement and fuel in the cement and bricks factories.
Graphical Abstract

Article Highlights
-
Comparison of biosorption ability of single (CA) and mixture (MA) of algae.
-
Pb(II) and Cu(II) chosen as model metal contaminants.
-
Different biosorption behavior of CA and MA.
-
Embodying isotherm data and kinetic data using different models.
-
Establishment of MA as superior biosorbent than CA for eradicating Cu(II) and Pb(II).





Similar content being viewed by others
References
Ahalya N, Ramachandra TV, Kanamadi RD (2003) Biosorption of heavy metals. Res J Chem Environ 7(4):71–79
Ahmadi Z, Esmaeili J, Kasaei J, Hajialioghli R (2018) Properties of sustainable cement mortars containing high volume of raw diatomite. Sustain Mater Technol 16:47–53
Akbari M, Hallajisani A, Keshtkar AR, Shahbeig H, Ghorbanian SA (2015) Equilibrium and kinetic study and modeling of Cu(II) and Co(II) synergistic biosorption from Cu(II)–Co(II) single and binary mixtures on brown algae C. indica. J Environ Chem Eng 3(1):140–149
Akpomie K, Dawodu FA (2015) Potential of a low-cost Bentonite for heavy metal abstraction from binary component system. Beni-Suef Univ J Basic Appl Sci 4(1):1–13
Al-Musawi TJ, Brouers F, Zarrabi M (2017) Kinetic modeling of antibiotic adsorption onto different nanomaterials using the Brouers–Sotolongo fractal equation. Environ Sci Pollut Res 24:4048–4057. https://doi.org/10.1007/s11356-016-8182-z
Al-Musawi TJ, Brouers F, Zarrabi M, Noroozi R (2018) What can the use of well-defined statistical functions of pollutants sorption kinetics teach us? A case study of cyanide sorption onto LTA zeolite nanoparticles. Environ Technol Innov 10:46–54
Brouers F (2014) The fractal (BSf) kinetics equation and its approximations. J Mod Phys 5:1594–1601
Brouers F, Al-Musawi TJ (2018) Brouers-Sotolongo fractal kinetics versus fractional derivative kinetics: a new strategy to analyze the pollutants sorption kinetics in porous materials. J Hazard Mater 350:162–168
Brouers F, Sotolongo-Costa O (2006) Generalized fractal kinetics in complex systems (application to biophysics and biotechnology). Phys A 368(1):165–175
Castro RSD, Caetano LR, Ferreira G, Padilha PM, Saeki MJ, Zara LF, Martines MAU, Castro GR (2011) Banana peel applied to the solid phase extraction of copper and lead from river water: preconcentration of metal ions with a fruit waste. Ind Eng Chem Res 50(6):3446–3451
Davis A, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metals biosorption by brown algae. Water Res 37:311–4330
Demirbas O, Karadag A, Alkan M, Dogan M (2008) Removal of copper ions from aqueous solutions by hazelnut shell. J Hazard Mater 153(1–2):677–684
Du J, Dong Z, Yang X, Zhao L (2018) Facile fabrication of sodium styrene sulfonate-grafted ethylene-vinyl alcohol copolymer as adsorbent for ammonium removal from aqueous solution. Environ Sci Pollut Res 25(27):27235–27244
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–407
Gisi SD, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Hamissa AMB, Brouers F, Mahjoub B, Seffen M (2007) Adsorption of textile dyes using Agave americana (L.) fibres: equilibrium and kinetics modelling. Adsorpt Sci Technol 25(5):311–325
Holan ZR, Volesky B (1995) Accumulation of cadmium, lead and nickel by fungal and wood biosorbents. J Appl Biochem Biotechnol 53:133–142
Hossain MA, Ngo HH, Guo WS, Setiadi T (2012) Adsorption and desorption of copper(II) ions onto garden grass. J Bioresour Technol 121:386–395
Hossam DM, Akasha RA (2007) Chemically modified algae residues as a new adsorbent for Pb(II) ions from aqueous solution. Energy Educ Sci Technol 19:17–36
Jelčić Z, Maduna K, Zrnčević S (2017) Kinetics of catalytic peroxide oxidation of phenol over three-dimensional fractals. Ind Eng Chem Res 56(45):12994–13009
Kesraoui A, Selmi T, Seffen M, Brouers F (2016) Influence of alternating current on the adsorption of indigo carmine. Environ Sci Pollut Res 24(11):9940–9950
Kongarapu RM, Nayak AK, Khobragade MU, Pal A (2018) Surfactant bilayer on chitosan bead surface for enhanced Ni(II) adsorption. Sustain Mater Technol 15:78. https://doi.org/10.1016/j.susmat.2018.e00077
Kowanga KD, Gatebe E, Mauti GO, Mauti EM (2014) Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu(II) and Pb(II) using defatted Moringa oleifera seed powder. J Phytopharmacol 5(2):71–78
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Mohammed A, Abed F, Al-Musawi TJ (2016) Biosorption of Pb(II) from aqueous solution by spent black tea leaves and separation by flotation. Desalin Water Treat 57(5):2028–2039
Mohammed AA, Samaka IS, Brouers F, Al-Musawi TJ (2018) Role of Fe3O4 magnetite nanoparticles used to coat bentonite in zinc(II) ions sequestration. Environ Nanotechnol Monit Manag 10:17–27
Mukherjee S, Dutta S, Ray S, Halder G (2018) A comparative study on defluoridation capabilities of biosorbents: isotherm, kinetics, thermodynamics, cost estimation, and ecotoxicological study. Environ Sci Pollut Res 25:17473–17489
Nocete F, Álex E, Nieto JM, Sáez R, Bayona MR (2005) An archaeological approach to regional environmental pollution in the south-western Iberian Peninsula related to Third millennium BC mining and metallurgy. J Archaeol Sci 32(10):1566–1576
Noroozi N, Al-Musawi T, Kazemian H, Kalhori HE, Zarrabi M (2018) Removal of cyanide using surface-modified Linde type-A zeolite nanoparticles as an efficient and ecofriendly material. Water Process Eng 21:44–51
Onundi YB, Mamun AA, Al Khatib MF, Ahmed YM (2010) Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int J Environ Sci Technol 7(4):751–758
Ratnasri PV, Hemalatha KPJ (2015) Studies on Biosorption of different metals by isolates of Aspergillus species. IOSR J Pharm Biol Sci (IOSR-JPBS) 10(5):01–05
Romera E, Gonzalez F, Ballester A, Blazquez MJ (2007) Comparative study of heavy metals using different types of algae. Bioresour Technol 98:3344–3353
Sari A, Tuzen M (2008) Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 157:448–454
Sarmah S, Saikia J, Bordoloi D, Goswamee RL (2017) Surface modification of paddy husk ash by hydroxyl-alumina coating to develop an efficient water defluoridation media and the immobilization of the sludge by lime-silica reaction. J Environ Chem Eng 5245(5):4483–4493
Singh A, Kumar D, Gaur JP (2007) Copper(II) and lead(II) sorption from aqueous solution by non-living Spirogyra neglecta. Bioresour Technol 98(18):3622–3629
Sopasakis P, Sarimveis H, Macheras P, Dokoumetzidis A (2018) Fractional calculus in pharmacokinetics. J Pharmacokinet Pharmacodyn 45(1):107–125
Sulaymon AH, Ebrahim SE, Al-Musawi TJ, Abdullah SM (2010) Removal of lead, cadmium, and mercury ions using biosorption. Iraqi J Chem Pet Eng 11:1–13
Sulaymon A, Mohammed A, Al-Musawi T (2013) Column biosorption of lead, cadmium, copper, and arsenic ions onto algae. J Bioprocess Biotech 3:128. https://doi.org/10.4172/2155-9821.1000128
Sulaymon A, Mohammed A, Al-Musawi T (2014) Comparative study of removal of cadmium(II) and chromium(III) ions from aqueous solution using low-cost biosorbent. Int J Chem React Eng 12(1):1–10
Tariq M, Durrani AI, Farooq U, Tariq M (2018) Efficacy of spent black tea for the removal of nitrobenzene from aqueous media. J Environ Manag 223:771–778
Temkin MJ, Pyzhey V (1940) Recent modification to Langmuir isotherm. Acta Physiochim 12:217–222
Turner BD, Henley BJ, Sleap SB, Sloan SW (2015) Kinetic model selection and the Hill model in geochemistry. Int J Environ Sci Technol 12:2545–2558
WHO (World Health Organization) (2004) Guidelines for drinking-water quality, vol 1, 3rd edn. World Health Organization, Geneva, p 595
Yan C, Li G, Xue P, Wei Q, Li Q (2010) Competitive effect of Cu(II) and Zn(II) on the biosorption of lead(II) by Myriophyllum spicatum. J Hazard Mater 179:721–728
Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater 174:137–143
Zhang Z, Bai R (2003) Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. J Colloid Interface Sci 264:30–38
Zheng W, Li XM, Wang F, Yang Q, Deng P, Zeng GM (2008) Adsorption removal of cadmium and copper from aqueous solution by areca—a food waste. J Hazard Mater 157:490–495
Acknowledgements
Authors express their gratitude to colleagues at the University of Baghdad (Baghdad, Iraq) and Isra University (Amman, Jordan) for their spiritual support at various stages of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdelkareem, H., Alwared, A., Al-Musawi, T.J. et al. A Comparative Study for the Identification of Superior Biomass Facilitating Biosorption of Copper and Lead Ions: A Single Alga or a Mixture of Algae. Int J Environ Res 13, 533–546 (2019). https://doi.org/10.1007/s41742-019-00194-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-019-00194-9