Abstract
The spatial and seasonal distribution of eight US Environmental Protection Agency priority poly aromatic hydrocarbons (PAHs); namely benzo(b)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, benzo(k)fluoranthene, chrysene, benzo(a)anthracene, dibenz(a,h)anthracene and fluoranthene were investigated in 35 soil samples. Gas chromatography with flame ionization detection was used for the quantification of the PAHs in the residential, roadside and industrial soil samples collected in proximity to an industrial area, including a petrochemical plant, in Cape Town. The sum of the concentrations of the detected PAHs (ΣPAHs) in winter ranged between nd (not detected) and 13.88 mg/kg, with a mean concentration of 4.08 mg/kg and a standard deviation of distribution of 2.64 mg/kg, while in summer, ΣPAHs ranged between nd and 10.43 mg/kg, with a mean concentration of 0.50 mg/kg and standard deviation of distribution 1.83 mg/kg. The presence and concentration level of the PAHs detected in the soil samples vary significantly under climatic change (p < 0.05). The maximum concentrations of individual PAHs in the soil samples were higher in winter compared to the maximum concentrations of the PAHs detected in summer. The diagnostic ratios of selected PAH compounds showed that petrogenic processes were the most likely sources of the PAH compounds in the soil samples. In summer and winter, the total PAH concentration increased in order of residential soil < industrial soil ≤ roadside soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution has become one of the most globally stressful challenges that mainly originate from industrialization processes, population growth and globalization (Khan and Ghouri 2011). For the past decades, persistent organic pollutants (POPs), i.e., polycyclic aromatic hydrocarbons (PAHs), polycyclic organic matter (POM), poly-nuclear aromatic hydrocarbons, poly-nuclear aromatics (PNAs) or poly-nuclear hydrocarbons, have been identified as potential environmental contaminants (ATSDR 2009). Based on their stability, the aforementioned class of compounds has been proved resistant to biodegradation. Their accumulation in living organisms and nature significantly impacts human health and the ecosystems (Allan et al., 2012). Apart from this, anthropogenic activities have also been held responsible for the presence of PAHs in the environment (Abdel-shafy and Mansour 2015). Literature maintained that the discharge of PAHs into the environment usually occurs through thermal processes which might involve complete/incomplete combustion of substances such as coal and similar activities including burning fossil fuel (e.g., wood, oil, and charcoal), garbage, charcoal meat, and heating of tobacco (Chen and Chen 2011). Sixteen PAHs have been considered on the priority list of pollutants by the US Environmental Protection Agency (USEPA 2000). The sixteen prioritized PAHs include naphthalene, acenaphthylene, acenaphthene, fluorine, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, benzo(ghi)perylene, indeno(1,2,3-cd)pyrene, and dibenz(a,h)anthracene.
According to El-Shahawi et al. (2010), the fate of PAHs is of great environmental concern due to their toxicity and tendency to accumulate and persist in different environmental matrices. However, this depends on several factors such as atmospheric photolysis, sorption, water and lipid solubility, chemical oxidation, vaporization, and microbial degradation (Wick et al. 2011). Furthermore, Wick et al. (2011) reported that the PAHs bind easily to organic materials which make them less susceptible to biodegradation. However, PAHs often degrade naturally in the soil; likewise, their high concentration could impede their decomposition process. In addition, natural and biological degradation processes are very slow because of the structure of the PAH molecules.
Humans, biological organisms, and the environment could be exposed to PAHs through different pathways such as inhalation, breathing of contaminated air during asphalt and coal tar production, in petroleum plants and smokehouses (Choosong et al. 2010). Exposure may also occur when the skin is in contact with contaminated water, air, and the soil which are closer to the hazardous sites (ATSDR 2009). Serious hazardous effects have been associated with exposure to PAHs which may be acute or chronic. The acute (short-term) effects include vomiting, eye irritation, nausea, diarrhoea, and confusion, while the chronic (long-term) effects include cancer, cataracts, kidney and liver damage, and skin inflammation. Therefore, it is crucial to assess and update data on the existence and occurrence of these harmful substances in Cape Town, South Africa.
Only a few environmental studies have been reported on the levels, presence and possible sources of PAHs in South Africa, despite the fact that it is one of the largest economies in Africa (Chimuka et al. 2015). A study conducted in Gauteng Province of South Africa, reported by Nieuwoudt et al. (2011) shows that the total concentrations of PAH in soil ranged from 44 to 39,000 ng/g.
Industrial activities, particularly petrochemical industries, are major hot spots for the release of PAHs into the environment. Some refining and production operations have been reported to release a substantial quantity of PAHs into soils and water bodies. These substantial quantities enter as process water effluents in nearby industrial sites and as gaseous particulates into the atmosphere (Das et al. 2008). In addition, there is a lack of critical environmental exposure levels that guarantee the safety and health of the environment in many African cities including Cape Town. It is, therefore, important to evaluate their occurrence and levels in different environmental matrices such as soil concentrations within the vicinity of potential sources.
Materials and methods
Description of the study area
This study was conducted in proximity to a major industrial area, which includes a petrochemical refinery, in Cape Town, a port city on South Africa’s southern west coast. The city with a population of about 3,750,000 people is located at latitude 33.55°S and longitude 18.25°E. Based on the world weather information service, Cape Town has a Mediterranean climate with a dry, warm summer and a mild, wet winter. Winter months in the city average a maximum of 18 °C and a minimum of 8 °C. On the other hand, summer months average a maximum of 26 °C and a minimum of 16 °C. The total annual rainfall in the city averages 515 mm. In winter the wind is generally strong and comes from the northwest, while in summer it varies between southeast and southwest.
Soil samples collection
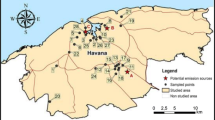
Thirty-five soil samples were collected in residential areas, roadsides and the industrial area in vicinity of a major industry in Cape Town (Fig. 1). The distribution of the sampling sites was as follows: 10 soil samples were collected close to the road, 19 soil samples were collected in the residential area and 6 in the industrial area. About 500 g of surface soil (0–5 cm depth) was collected. The soil samples were stored according to standard methods (ASTM D 4547).
Chemicals, solvents and materials
High purity solvents including n-hexane (99.5%), dichloromethane (99.5%), acetone (99.8%), and acetonitrile (99.9%) were purchased from Sigma-Aldrich (Germany). The PAHs, benzo(b)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, benzo(k)fluoranthene, chrysene, benzo(a)anthracene, dibenz(a,h)anthracene, and fluoranthene were purchased from Sigma-Aldrich (Germany). Sodium sulphate was purchased from Merck (South Africa), silica gel, and florisil were purchased from Merck (Germany). All glasswares used in this work were washed with soap and soaked in a prepared nitric acid solution for 24 h and then washed with distilled water and oven-dried, the glasswares were washed again with acetone before use.
Instrumentation and chromatographic conditions
A gas chromatograph (GC-2010 plus Shimadzu) equipped with a flame ionization detector (GC-FID) and a data processor was used for separation and determination of the eight PAHs. Parameters such as carrier gas flow rate, injection volume, oven temperature program parameter, flame ionization detectors temperature, column temperature, and split injection time and make-up gas flow rate, were optimized before analysis. An HP-5% phenyl methyl siloxane column (30 m length and 0.32 mm ID 0.25 μm film thickness) was used for separation of the analytes. The injection volume was 1.0 μL. The injector temperature was set at 270 °C and used as split mode and the split ratio was 1. The detector temperature was set at 320 °C. Helium was used as the carrier gas and make-up gas at a flow of 30 mL/min at a pressure of 200 kPa. Hydrogen flow rate was 40 mL/min; air flow rate was 400 mL/min. The column flow was 1.99 mL/min, linear velocity was 46.8 cm/s, purge flow was 3 mL/min. The oven program was 40 °C for 5 min, 200 °C hold for 10 min. The column temperature program was 170 °C hold for 4 min, a ramp to 250 °C at 15 °C/min with 5 min hold time and a final ramp to 300 °C at 10 °C/min with a 5 min hold time. The total run time was 24.33 min.
Physicochemical parameter analysis
The soil pH was determined using the ASTM D 4972 method. 5 g of soil sample was weighed out into a 50 mL beaker, followed by the addition of 10 mL of distilled water and two drops of 1 M CaCl2. The mixture was stirred in the shaker for 30 min. The mixture was then allowed to settle for 20 min. The pH of the supernatant was measured and recorded using a calibrated pH metre (Crison GLP 21+, Lasec, South Africa). The soil organic content was determined using the ASTM D 2974 method in which 1 g of air-dried soil was burnt in a furnace at 440 °C for 24 h. The soil sample was reweighed after burning to obtain the mass of burnt soil. The organic matter was determined by the difference in the mass of the burnt soil from the mass of the dried soil, expressed as a percentage.
Quantification of PAHs
Soil samples were extracted following the EPA microwave-assisted extraction method 3546. 10 g of the soil sample was weighed in triplicate and transferred into a beaker, followed by the addition of a mixture of 5 mL dichloromethane and 20 mL hexane. The mixtures were stirred for 2 h in the shaker to equilibrate. The recommended extraction conditions used were: temperature: 100 °C, pressure: 150 psi, time at temperature: 20 min, cooling: to room temperature, filtering/rinsing: with same solvent system. After extraction, the extracts were centrifuged at room temperature for 20 min at 2000 rpm to separate the solid phase and liquid phase.
Sample extracts were cleaned prior to analysis due to the presence of a large number of contaminants which could damage the GC column. In addition, the cleanup process minimizes interference during analysis. The column was prepared as follows: Cotton was stocked to the bottom of the column and then filled with 1 g of silica gel, 0.5 g of florisil followed by another 1 g of silica gel and then topped with 0.25 g sodium sulphate anhydrous. Each column was pre-washed and activated by eluting 20 mL of 4:1 mixture of n-hexane and dichloromethane at a flow rate of 1.0 mL/min. The concentrated extract was then loaded onto the column and eluted with about 80 mL of a mixture of n-hexane and dichloromethane. The volume of the eluates was brought to 2 mL using a rotary evaporator under reduced pressure.
Quality control
The test of reliability on PAHs concentration data was checked against spiked soil samples in recovery studies carried out in replicate. The results obtained showed procedural consistencies in selected PAH standard extraction from the spiked soils. The extraction efficiency of the selected PAHs obtained from 20 g soil samples ranged from 93.02 to 100.04%. The limit of detection and limit (LOD) (defined as S/N > 3) of individual PAHs, were between 0.0006 and 0.0023 mg/kg.
Statistical analysis
The results were performed using the statistical software package SAS system version 9.4. Samples in which the PAHs were not detected (nd) were assumed to be equal one-half of the detection limit (nd = 1/2 LOD). The correlation between variables was performed by the Spearman’s test. A multivariate analysis of the results was carried out using principal component analysis (PCA). The number of components extracted from the variables was determined according to Kaiser’s rule, which retains only components with Eigenvalue >1. Statistical significance of the difference was determined by the one-way analysis of variance (ANOVA) for the data that are normally distributed and for data that are not normally distributed. The Kruskal–Wallis test was used for all statistical tests, a significance level of 0.05 was used (p < 0.05).
Results and discussion
Effect of soil organic matter (SOM) and pH on the PAH concentrations in soil samples
The percentage SOM in the 35 soil samples ranged from 0.10 to 8.77% with a mean value of 2.74% and from 0.20 to 7.61% with a mean value of 2.91% in winter and summer, respectively. In summer, a weak correlation (r s ≤ 0.39) was found between SOM and the PAH concentrations. On the other hand, a moderate positive correlation (r s values between 0.40 and 0.59) was observed between SOM and Chry (r s = 0.47) and BbF (r s = 0.42) during winter. A weak correlation between the SOM and PAH concentrations in summer suggests that soil samples were perhaps contaminated to different levels, probably due to easy degradation of the PAHs. Another possible reason can be due to the fact that the PAH emission point source could be low even if the SOM is high (Wang et al. 2012). However, the moderate correlation in winter can be explained by the fact that during the winter season, PAH degradation is expected to slow down. These results are consistent with findings reported in other studies conducted (Cai et al. 2007). Wang et al. (2010) reported that a very weak correlation should be expected between SOM and PAHs concentration in a soil subjected by continuous input of fresh PAH contamination.
Soil pH is an important factor controlling the mobility and accumulation of organic substances such as PAHs in the soils. The pH values from the 35 sampling sites ranged from 4.63 to 7.79 with a mean value of 7.28 and from 6.07 to 8.20 with a mean value of 6.67 in winter and summer, respectively. In summer, a weak positive correlation was found between pH and PAH concentrations (r s ≤ 0.39), while in winter a moderate negative correlation exists for Fluo (r s = −0.45) and Chry (r s = −0.41). The remaining PAHs showed a weak correlation. A weak correlation indicates that the pH did not have a significant influence in the distribution of the PAH compounds in the soil.
Concentration of PAHs soil samples
The concentration levels of the investigated PAHs in the different soil samples were variable. The PAHs Fluo, BaP, BkF, Chry, IP, BbF, DahA, and BaA were detected in the soil samples collected from the different sampling sites around Cape Town. The mean concentrations, standard deviations of distribution, minimum and maximum values for the concentrations of the selected PAHs detected in soil collected in the vicinity of a petrochemical plant in Cape Town during winter and summer are presented in Table 1.
The sum of the concentrations of the detected PAHs (ΣPAHs) ranged between nd and 13.88 mg/kg, with a mean concentration of 4.08 mg/kg and a standard deviations of distribution of 2.64 mg/kg in winter while in summer ΣPAHs ranged between nd and 10.43 mg/kg, with a mean concentration of 0.50 mg/kg and standard deviation of distribution 1.83 mg/kg. In winter the mean concentration of the individual PAH compounds ranged from nd to 1.64 mg/kg with DahA not detected and IP having the highest mean value (1.64 mg/kg) while in summer, the mean concentration of the individual PAHs range from nd to 0.15 mg/kg with BaA not detected and IP having the highest mean value (0.15 mg/kg). Benzo(a)anthracene was not detected in winter. This may be attributed to the physical and chemical properties of the PAH. On the other hand, dibenz(a,h)anthracene was not detected in summer. This could perhaps be as a result of the tendency for volatilization of dibenz(a,h)anthracene from the soil during the warm season, occasioned by an increase in temperature.
All the individual PAH compounds detected in soil samples except for fluoranthene were above the maximum allowable concentration (MACs) recommended by ATSDR (2009). A significant number of sites had levels for IP and BaP exceeding allowed limits (Table 2). This could be ascribed to the fact that these PAHs exhibit the lowest vapour pressure.
The regulatory limit for the amount of carcinogenic and toxic polycyclic aromatic hydrocarbons in soils are 3.00, 0.15, 1.50, 0.01, 1.50, 0.15, 0.15, 0.01 mg/kg for Fluo, IP, Chry, BaP, BkF, BaA, BbF, and DahA, respectively. These results are consistent with findings reported in the study conducted by Nieuwoudt et al. (2011) in central South Africa. The author reported that the total PAH concentration of soil varied from 0.04 to 39.00 mg/kg. In the study conducted in urban soils of UK, the sum of the concentrations of the detected PAHs ranged from 4 to 67 mg/kg, with a mean concentration of 18 mg/kg (Vane et al. 2014). Similarly, Peng et al. (2011) reported the total concentration of PAHs in the soils of Beijing ranged from 0.09 to 13.14 mg/kg with a mean concentration of 1.23 mg/kg.
Seasonal effects on concentration levels of PAHs in soil samples
The presence and concentration of the PAHs detected in soil samples vary significantly under climatic change (p < 0.05). The maximum concentrations of individual PAHs in soil samples were higher in winter while in summer most of the individual PAHs were not detected in most of the sampling sites (Fig. 2).
The results are consistent with the findings of Kumar et al. (2015) who reported that the concentration of PAHs detected in soil samples were higher in the winter season and lower in the summer season. In this study, the summer was characterized by an average temperature ranging from 14.2 to 25.4 °C with an average precipitation of 20 mm. The above temperature might have favoured volatilization of the PAHs from soil to another media as well as the biodegradation of the PAHs in the soil. Moreover, at elevated temperature, photochemical decomposition rates of the PAHs in the atmosphere increases and could result in decreased deposition of the pollutant on the soil surface (Khillare et al. 2014). On the other hand, during winter the season was characterized by an average temperature ranging from 7.5 to 17.8 °C with an average precipitation of 82 mm. According to Ahangar (2010), the microbial breakdown of PAHs is decreased during winter which might have contributed to the higher concentration of PAHs during this season. In addition, dry deposition and wet deposition of PAHs on the soil surface might have also contributed to the level of PAHs in soil. According to Khillare et al. (2014), the higher concentration of PAHs in the winter season could also be attributed to higher emissions (biomass and coal burning) leading to higher rate of deposition of PAHs onto the soil.
Seasonal variation and spatial distribution of PAH concentrations in soil samples
The PAH compounds in the soils varied from one sampling site to another. Fluo, BaP, IP, Chry, BaA, BkF, and BbF were detected in 51, 86, 46, 97, 17, 83, and 9% of the soil samples in winter, respectively, while in summer DahA, Fluo, BaP, IP, BbF, BkF, and Chry were detected in only about 25, 6, 14, 26, 14, 8, and 6%, respectively. The decrease in the presence of the PAHs may be attributed to the increase of volatilization of the PAHs from the soil during summer. This was consistent with the findings of Masih and Taneja (2006) who reported that the maximum mean concentration of the PAH compounds was higher in the soil samples during the winter season and decreased in the summer season.
The concentration of IP, BaP and BkF were higher at all the sampling sites during winter compared to the other PAHs. This can probably be explained by the fact that they are more abundant in the environment. The sum of the PAHs at the road side in winter ranged from 4.43 to 9.31 mg/kg, from 2.31 to 5.37 mg/kg for the soil samples collected at the industrial area and from 1.32 to 5.23 mg/kg for the soil samples collected at the residential area. On the other hand, the soil samples collected at the road side during summer ranged from nd to 6.27 mg/kg from nd to 8.65 mg/kg for the soil samples collected at the industrial area and from nd to 3.41 mg/kg for the soil samples collected at the residential area. The total PAH concentration increased in order of residential soil < industrial soil ≤ roadside soil.
From the spatial distribution, it was observed that residential areas located in the northwest of the petrochemical plant contributed least PAH. The highest PAH concentration at the road side suggested that vehicular emissions probably contribute the most to the level of the PAHs in the soils followed by the industrial emissions during both winter and summer. These results are in agreement with the finding of Rafael et al. ( 2014) who reported that the levels of the PAHs in a vicinity of a petrochemical plant were due to vehicular exhausts from both diesel and gasoline engines. Similarly, results were also reported by Wang et al. (2013) where the highest concentrations of the PAHs were observed at the roadsides (0.96 mg/kg), followed by industrial areas (0.71 mg/kg) and residential areas (0.52 mg/kg). The spatial distribution of the PAH compounds showed that the concentrations of PAH at some sites were lower close to the petrochemical plant. Wind direction and speed were found to affect the spatial distribution and accumulation of PAHs in the terrestrial environment (Nam et al. 2003). In Cape Town, the wind is predominantly from the northwest in winter while in summer it varies between southeast and southwest. Most locations with higher concentrations were located in southwest and southeast areas of the petrochemical plant and the locations with lower PAH concentrations were mainly distributed near the northwest area of the petrochemical plant. Those results suggested that the distribution of PAHs could possibly be affected by the wind direction.
Principal component analysis
To further demonstrate the seasonal variation and spatial distribution in the concentration of the PAHs in soil samples an analysis based on the principal component method (PCA) was employed. Figure 3 gives a visual representation of the seasonal variations and spatial.
PCA accounted for 62% of the variance. The clustering of winter and summer sites in the biplot suggests a seasonal effect on the PAHs concentrations in the soil samples. It is also evident that pH weakly correlates with summer PAH levels, with no correlation in winter. Similarly, a SOM appears to have no effect on either summer or winter PAH levels.
Probable sources of PAHs in soil in a vicinity of a petrochemical plant
The potential sources of PAHs in different environmental media can be identified using the ratios between pairs of the concentration of individuals PAHs. The literature values presented in Table 3 were used to characterize possible sources of the PAHs in this study.
The ratio of BaP/(BaP + Chry) and the possible sources of PAHs in the 35 soil samples during winter are presented in Table 3. In winter, BaP/(BaP + Chry) ratio ranged from 0.66 to 0.78. About 97% of the sampling sites exhibited the typical attribute of petrogenic combustion. In summer, the ratio BaP/(BaP + Chry) could not be calculated because benzo(a)pyrene and chrysene were not detected in most of the sampling sites presumably due to the higher rate of degradation of the PAHs.
Conclusion
All of the selected PAHs were present in the soil samples. Indeno(1,2,3-cd)pyrene and benzo(a)anthracene exceeded the maximum allowed concentration for PAHs in soils at almost all the sampling sites during both seasons. Fluoranthene and benzo(k)fluoranthene levels were within the MAC at all the sampling sites. A seasonal influence on the PAH levels is observed with the higher soil concentration in winter (p < 0.05). The observed trend for the concentration of the eight PAHs for winter was; residential soils (5.23 mg/kg) < industrial (5.37 mg/kg) < roadside soils (9.31 mg/kg). The corresponding trend for summer was; residential soils (3.41 mg/kg) < roadside soils (6.27 mg/kg) < industrial soils (8.65 mg/kg). The data suggest that vehicular traffic could be a significant contributor to PAH level in the environment.
References
Abdel-Shafy HI, Mansour MS (2015) A review on polycyclic aromatic hydrocarbons: source, environmental impact effect on human health and remediation. Egypt J Petrol 25:107–123
Agency for Toxic Substances and Disease Registry (ATSDR) (2009) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs), Atlanta, GA: US Department of Health and Human Services, Public Health Service. http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf. Accessed on 22 Sept 2015
Ahangar AG (2010) Sorption of PAHs in the soil environment with emphasis on the role of soil organic matter: a review. World Appl Sci J 11:759–765
Allan SE, Smith BW, Anderson KA (2012) Impact of the Deepwater Horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ Sci Technol 46:2033–2039
Cai QY, Mo CH, Li YH, Zeng QY, Katsoyiannis A, Wu QT, Férard JF (2007) Occurrence and assessment of polycyclic aromatic hydrocarbons in soils from vegetable fields of the Pearl River Delta South China. Chemosphere 68:159–168
Chen CW, Chen CF (2011) Distribution, origin, and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor Taiwan. Mar Poll Bullet 63:417–423
Chimuka L, Sibiya P, Amdany R, Cukrowska E, Forbes PBC (2015) Status of PAHs in environmental compartments of South Africa: a country report. Poly Aromat Comp 36:1–19
Choosong T, Chomanee J, Tekasakul P, Tekasakul S, Otani Y, Hata M, Furuuchi M (2010) Workplace environment and personal exposure of PM and PAHs to workers in natural rubber sheet factories contaminated by wood burning smoke. Aeros Air Qual Res 10:8–21
Das SK, Routh J, Roychoudhury AN (2008) Sources and historic changes in polycyclic aromatic hydrocarbon input in a shallow lake Zeekoevlei South Africa. Org Geochem 39:1109–1112
El-Shahawi MS, Hamza A, Bashammakh AS, Al-Saggaf WT (2010) An overview on the accumulation distribution transformations toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 80:1587–1597
Khan MA, Ghouri AM (2011) Environmental pollution: its effects on life and its remedies. J Arts Sci Commun 2:276–285
Khillare PS, Hasan A, Sarkar S (2014) Accumulation and risks of polycyclic aromatic hydrocarbons and trace metals in tropical urban soils. Environ Monit Assess 186:2907–2923
Kumar V, Kothiyal NC, Saruchi (2015) Analysis of polycyclic aromatic hydrocarbon toxic equivalency factor and related carcinogenic potencies in roadside soil within a developing city of Northern India. Poly Aromat Comp 36:506–526
Masih A, Taneja A (2006) Polycyclic aromatic hydrocarbons (PAHs) concentrations and related carcinogenic potencies in soil at a semi-arid region of India. Chemosphere 65:449–456
Nam JJ, Song BH, Eom KC, Lee SH, Smith A (2003) Distribution of polycyclic aromatic hydrocarbons in agricultural soils in South Korea. Chemosphere 50:1281–1289
Nieuwoudt C, Pieters R, Quinn LP, Kylin H, Borgen AR, Bouwman H (2011) Polycyclic aromatic hydrocarbons (PAHs) in soil and sediment from industrial residential and agricultural areas in Central South Africa: an initial assessment. Soil Sed Contam 20:188–204
Peng C, Chen W, Liao X, Wang M, Ouyang Z, Jiao W, Bai Y (2011) Polycyclic aromatic hydrocarbons in urban soils of Beijing: status sources distribution and potential risk. Environ Pollut 159:802–808
Rafael LO, Luciana V, Graciela A (2014) Characterization of polycyclic aromatic hydrocarbon levels in the vicinity of a petrochemical complex located in a densely populated area of the Rio de Janeiro Brazil. Atmos Pollut Res 5:87–95
USEPA (2000) Toxic Release Inventory Public Data Release. Office of Environmental Information, United States Environmental Protection Agency, Washington, DC. http://www.epa.gov/triinter/tridata/index.htm. Accessed on 10 Aug 2015
Vane CH, Kim AW, Beriro DJ, Cave MR, Knights K, Moss-Hayes V, Nathanail PC (2014) Polycyclic aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB) in urban soils of Greater London, UK. Appl Geochem 51:303–314
Wang W, Simonich SLM, Xue M, Zhao J, Zhang N, Wang R, Cao J, Tao S (2010) Concentrations sources and spatial distribution of polycyclic aromatic hydrocarbons in soils from Beijing Tianjin and surrounding areas North China. Environ Pollut 158:1245–1251
Wang X, Zuo Q, Duan Y, Liu W, Cao J, Tao S (2012) Factors affecting spatial variation of polycyclic aromatic hydrocarbons in surface soils in North China Plain. Environ Toxicol Chem 31:2246–2252
Wang XT, Miao Y, Zhang Y, Li YC, Wu MH, Yu G (2013) Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: occurrence source apportionment and potential human health risk. Sci Tot Environ 447:80–89
Wick AF, Haus NW, Sukkariyah BF, Haering KC, Daniels WL (2011) Remediation of PAH-contaminated soils and sediments: a literature review. Environ Soil Sci:102
Acknowledgements
We acknowledge financial support from the University Research Fund (Cape Peninsula University of Technology). The Government of Gabon for the research scholarship awarded. Thanks to Zandile Mthembu and Gillian Fennessy-Yon for their support on the GC and Dr O. Olatunji for the invaluable support with the analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Omores, R.A., Wewers, F., Ikhide, P.O. et al. Spatio–Temporal Distribution of Polycyclic Aromatic Hydrocarbons in Urban Soils in Cape Town, South Africa. Int J Environ Res 11, 189–196 (2017). https://doi.org/10.1007/s41742-017-0018-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-017-0018-2