Abstract
Objective
Periodontitis is an infectious oral disease characterized by periodontal pocket, clinical attachment loss and alveolar bone loss. Smoking has a negative impact on the outcome of periodontal treatment, and low-level laser therapy (LLLT) as an adjunct to scaling and root planing (SRP) has shown to enhance wound healing by biostimulatory action on various cells, increasing angiogenesis and release of growth factors. Transforming growth factor beta 1 (TGF-β1) possesses both proinflammatory and anti-inflammatory characteristics and plays a role in tissue remodeling and tissue regeneration. This study was undertaken to evaluate the effect of LLLT as an adjunct to SRP on healing by evaluating clinical parameters—gingival index (GI), plaque index (PI), probing depth (PD), clinical attachment level (CAL) and TGF-β1 in the Gingival Crevicular Fluid (GCF) of nonsmokers and smokers with periodontitis.
Materials and methods
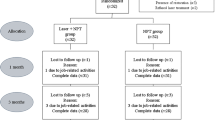
A total of 30 patients with periodontitis were selected and further sub divided into Group I (15 patients)—smokers with periodontitis and Group II (15 patients)—nonsmokers with periodontitis. The GCF was collected to analyze TGF-β1 levels on day 1, day 7 and day 30. Clinical parameters such as GI, PI, PD and CAL were assessed on day 1 and day 30.
Result
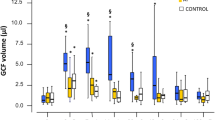
The TGF-β1 levels decreased post treatment in both the groups with a good reduction seen in nonsmokers when compared to smokers. All the clinical parameters (GI, PI, PD and CAL) reduced from day 1 to day 30 with statistical significance (p = 0.001) in both the groups. There was a statistically significant reduction in the TGF-β1 levels in both the groups at all time intervals.
Conclusion
In the present study the TGF-β1 was present in all the three GCF samples that were collected on day 1 and on day 7 and day 30 post SRP and LLLT. A higher level of TGF-β1 was noticed on day 1 (before treatment) that gradually reduced after SRP and LLLT in both smokers and nonsmokers. In addition to the regular wound healing, the addition of LLLT increases neovascularization and enhances healing after SRP, which may prove beneficial in smokers.
Similar content being viewed by others
References
Javed F, Al-Rasheed A, Almas K, Romanos GE, Al-Hezaimi K (2012) Effect of cigarette smoking on the clinical outcomes of periodontal surgical procedures. Am J Med Sci 343(1):78–84
Chaffee BW, Couch ET, Vora MV, Holliday RS (2021) Oral and periodontal implications of tobacco and nicotine products. Periodontol 2000 87(1):241–253
Tatsumi M, Yanagita M, Yamashita M, Hasegawa S, Ikegami K, Kitamura M et al (2021) Long-term exposure to cigarette smoke influences characteristics in human gingival fibroblasts. J Periodontal Res 56(5):951–963
Trombelli L, Kim CK, Zimmerman GJ, Wikesjö UM (1997) Retrospective analysis of factors related to clinical outcome of guided tissue regeneration procedures in intrabony defects. J Clin Periodontol 24(6):366–371
Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL (1994) The effect of smoking on the response to periodontal therapy. J Clin Periodontol 21(2):91–97
Talonpoika JT, Hämäläinen MM (1992) Collagen III aminoterminal propeptide in gingival crevicular fluid before and after periodontal treatment. Scand J Dent Res 100(2):107–110
Buduneli N, Kütükçüler N, Aksu G, Atilla G (2001) Evaluation of transforming growth factor-beta 1 level in crevicular fluid of cyclosporin A-treated patients. J Periodontol 72(4):526–531
Gürkan A, Emingil G, Cinarcik S, Berdeli A (2006) Gingival crevicular fluid transforming growth factor-beta1 in several forms of periodontal disease. Arch Oral Biol 51(10):906–912
Convissar RA (2011) Principles and practice in laser dentistry, 1st edn. Mo Mosby Elsevier, St. Louis
Castro GL, Gallas M, Núñez IR, Borrajo JL, Varela LG (2006) Histological evaluation of the use of diode laser as an adjunct to traditional periodontal treatment. Photomed Laser Surg 24(1):64–68
Silveira LB, Prates RA, Novelli MD, Marigo HA, Garrocho AA, Amorim JC et al (2008) Investigation of mast cells in human gingiva following low-intensity laser irradiation. Photomed Laser Surg 26(4):315–321
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol 89(Suppl 1):S159–S172
Pamuk F, Lütfioğlu M, Aydoğdu A, Koyuncuoglu CZ, Cifcibasi E, Badur OS (2017) The effect of low level laser therapy as an adjunct to nonsurgical periodontal treatment on gingival crevicular fluid levels of transforming growth factor-beta 1, tissue plasminogen activator and plasminogen activator inhibitor 1 in smoking and nonsmoking chronic periodontitis patients: a split-mouth, randomized control study. J Periodontal Res 52(5):872–882
Arunachalam LT, Sudhakar U, Janarthanam AS, Das NM (2014) Effect of low level laser therapy on revascularization of free gingival graft using ultrasound Doppler flowmetry. J Indian Soc Periodontol 18(3):403–407
Qadri T, Miranda L, Tunér J, Gustafsson A (2005) The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol 32(7):714–719
Lai SM, Zee KY, Lai MK, Corbet EF (2009) Clinical and radiographic investigation of the adjunctive effects of a low-power He-Ne laser in the treatment of moderate to advanced periodontal disease: a pilot study. Photomed Laser Surg 27(2):287–293
Silverstein B (1982) Cigarette smoking, nicotine addiction, and relaxation. J Pers Soc Psychol 42(5):946–950
Han B, Emingil G, Özdemir G, Tervahartiala T, Vural C, Atilla G, Baylas H, Sorsa T (2012) Azithromycin as an adjunctive treatment of generalized severe chronic periodontitis: clinical, microbiologic, and biochemical parameters. J Periodontol 83(12):1480–1491
Aykol G, Baser U, Maden I, Kazak Z, Onan U, Tanrikulu-Kucuk S, Ademoglu E, Issever H, Yalcin F (2011) The effect of low-level laser therapy as an adjunct to nonsurgical periodontal treatment. J Periodontol 82(3):481–488
Tomasi C, Schander K, Dahlén G, Wennström JL (2006) Short-term clinical and microbiologic effects of pocket debridement with an Er:YAG laser during periodontal maintenance. J Periodontol 77(1):111–118
Vikram V, Ramakrishnan T, Anilkumar K, Ambalavanan N (2015) Changes in transforming growth factor-β1 in gingival crevicular fluid of patients with chronic periodontitis following periodontal flap surgery. J Clin Diagn Res. 9(2):ZC13-6
Sarahrudi K, Thomas A, Mousavi M, Kaiser G, Köttstorfer J, Kecht M, Hajdu S, Aharinejad S (2011) Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury 42(8):833–837
Matarese G, Isola G, Anastasi GP, Favaloro A, Milardi D, Vermiglio G, Vita G, Cordasco G, Cutroneo G (2012) Immunohistochemical analysis of TGF-β1 and VEGF in gingival and periodontal tissues: a role of these biomarkers in the pathogenesis of scleroderma and periodontal disease. Int J Mol Med 30(3):502–508
Saadeh PB, Mehrara BJ, Steinbrech DS, Dudziak ME, Greenwald JA, Luchs JS, Spector JA, Ueno H, Gittes GK, Longaker MT (1999) Transforming growth factor-beta1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am J Physiol 277(4):C628–C637
Steinsvoll S, Halstensen TS, Schenck K (1999) Extensive expression of TGF-beta1 in chronically-inflamed periodontal tissue. J Clin Periodontol 26(6):366–373
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balakrishnan, A., Arunachalam, L.T. & Sudhakar, U. Evaluation of TGF-β1 in gingival crevicular fluid and clinical parameters of smoker and nonsmoker patients treated with low-level laser therapy as an adjunct to scaling and root planing. Laser Dent Sci 6, 55–61 (2022). https://doi.org/10.1007/s41547-022-00147-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-022-00147-0