Abstract
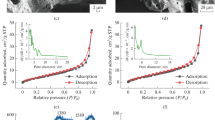
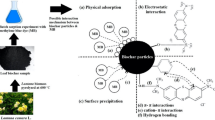
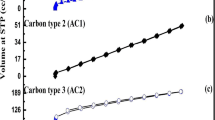
In this study, Ricinus communis stem (RCS) was chemically activated with potassium carbonate (K2CO3) and heated at 300 °C to produce low-temperature Ricinus communis stem-activated biochar (RCSB). These two biosorbents (RCS and RCSB) were used for the removal study of methylene blue (MB) and malachite green (MG) preferentially from synthetic aqueous solutions. Various analytical techniques were used to explore the surface morphology, surface area, crystallinity, elemental composition, and functional groups of natural (RCS) and potassium carbonate-activated biochar RCSB. Adsorption experiments were performed to evaluate the effect of pH, adsorbent dosage, contact time, initial dye concentration, stirring speed, particle size, and temperature. Initially, adsorption experiments were carried out with different nine cationic and anionic dyes. Among these dyes, two cationic dyes, MG and MB, show good dye removal %, RCS (~87%), and RCSB (~98%). The adsorption capacities qe (mg g−1) of all four systems are MB-RCSB: 242 mg g−1, MG-RCSB: 240 mg g−1, MB-RCS: 195 mg g−1, and MG-RCS: 190 mg g−1, respectively. The kinetics analysis data were best fitted with the intraparticle diffusion kinetics model (R2 = 1) and the isotherm analysis fits well with the Langmuir isotherm model. The thermodynamic parameters support the feasible, spontaneous, and exothermic adsorption in the temperature range of 293–323 K. Desorption experiments were carried out at pH 2 with an efficiency of ~97%. A regeneration study indicates biosorbents can be recycled up to five cycles. The present studies suggest using RCS as eco-friendly removal of MG and MB dyes up to 99%. Regarding the novelty of the work, the biochar reported in the present work was synthesized at a low temperature (300 °C) compared to the other higher temperature reported methods which are more economical. A cost estimation study reveals that two tons of dye-contaminated wastewater can be treated at a cost of approximately 152 INR.











Similar content being viewed by others
Data Availability
The data supporting this study’s findings are available on request from the corresponding author.
Code Availability
Not applicable.
References
Li W, Yang X, Doshmanziari M, Esmaeili H (2022) Elimination of methyl violet 2B dye from water using Citrus limetta leaves-activated carbon modified by copper-ferrite nanoparticles. Sep Sci Technol 57:509–522. https://doi.org/10.1080/01496395.2021.1919143
Hynes NRJ, Kumar JS, Kamyab H, Sujana JAJ, Al-Khashman OA, Kuslu Y, Ene A, Suresh Kumar B (2020) Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector—a comprehensive review. J Clean Prod 272:122636. https://doi.org/10.1016/j.jclepro.2020.122636
Sutar S, Patil P, Jadhav J (2022) Recent advances in biochar technology for textile dyes wastewater remediation: a review. Environ Res 209:112841. https://doi.org/10.1016/J.ENVRES.2022.112841
Zhu HY, Jiang R, Fu YQ, Jiang JH, Xiao L, Zeng GM (2011) Preparation, characterization and dye adsorption properties of γ-Fe2O3/SiO2/chitosan composite. Appl Surf Sci 258:1337–1344. https://doi.org/10.1016/j.apsusc.2011.09.045
Singh H, Chauhan G, Jain AK, Sharma SK (2017) Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. J Environ Chem Eng 5:122–135. https://doi.org/10.1016/j.jece.2016.11.030
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J Colloid Interface Sci 343:463–473. https://doi.org/10.1016/j.jcis.2009.11.060
Aguilar-Rosero J, Urbina-López ME, Rodríguez-González BE, León-Villegas SX, Luna-Cruz IE, Cárdenas-Chávez DL (2022) Development and characterization of bioadsorbents derived from different agricultural wastes for water reclamation: a review. Appl Sci 12. https://doi.org/10.3390/app12052740
Amalina F, Razak ASA, Krishnan S, Zularisam AW, Nasrullah M (2022) Dyes removal from textile wastewater by agricultural waste as an absorbent — a review. Clean Waste Syst 3:100051. https://doi.org/10.1016/j.clwas.2022.100051
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions — a review. Bioresour Technol 99:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Sharma P, Laddha H, Agarwal M, Gupta R (2022) Selective and effective adsorption of malachite green and methylene blue on a non-toxic, biodegradable, and reusable fenugreek galactomannan gum coupled MnO2 mesoporous hydrogel. Microporous Mesoporous Mater 338:111982. https://doi.org/10.1016/j.micromeso.2022.111982
Abate GY, Alene AN, Habte AT, Getahun DM (2020) Adsorptive removal of malachite green dye from aqueous solution onto activated carbon of Catha edulis stem as a low cost bio-adsorbent, Environ. Syst Res 9:1–13
Liu A, Zhou W, Shen K, Liu J, Zhang X (2015) One-pot hydrothermal synthesis of hematite-reduced graphene oxide composites for efficient removal of malachite green from aqueous solution. RSC Adv 5:17336–17342. https://doi.org/10.1039/c4ra15589k
Culp SJ, Beland FA (1996) Malachite green: a toxicological review. Int J Toxicol 15:219–238. https://doi.org/10.3109/10915819609008715
CHEN ZL, ZHANG JQ, HUANG L, YUAN ZH, LI ZJ, LIU MC (2019) Removal of Cd and Pb with biochar made from dairy manure at low temperature. J Integr Agric 18:201–210. https://doi.org/10.1016/S2095-3119(18)61987-2
Zhang J, Liu J, Liu R (2015) Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour Technol 176:288–291. https://doi.org/10.1016/j.biortech.2014.11.011
Safaei Khorram M, Zhang Q, Lin D, Zheng Y, Fang H, Yu Y (2016) Biochar: a review of its impact on pesticide behavior in soil environments and its potential applications. J Environ Sci (China) 44:269–279. https://doi.org/10.1016/j.jes.2015.12.027
Zhao N, Yang X, Zhang J, Zhu L, Lv Y (2017) Adsorption mechanisms of dodecylbenzene sulfonic acid by corn straw and poplar leaf biochars. Mater (Basel) 10. https://doi.org/10.3390/ma10101119
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sust Energ Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183. https://doi.org/10.1016/j.jenvman.2012.08.028
Jibril M, Noraini J, Poh LS, Evuti AM Removal of colour from waste water using coconut shell activated carbon (CSAC) and commercial activated carbon (CAC). J. Teknol. Sciences Eng 60(2013):15–19. https://doi.org/10.11113/jt.v60.1435
Garba A, Basri H, Nasri NS, R. (2016) Isma’il, synthesis and characterization of porous carbon from biomass using KOH and K2CO3 chemical activation, ARPN. J Eng Appl Sci 11:1613–1617
Akkari I, Graba Z, Bezzi N, Vithanage M, Kaci MM (2022) New insights into the effective removal of Basic Red 46 onto activated carbon produced from pomegranate peels. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03401-4
Akkari I, Graba Z, Bezzi N, Kaci MM, Merzeg FA, Bait N, Ferhati A, Dotto GL, Benguerba Y (2022) Effective removal of cationic dye on activated carbon made from cactus fruit peels: a combined experimental and theoretical study. Environ Sci Pollut Res:3027–3044. https://doi.org/10.1007/s11356-022-22402-4
Graba Z, Akkari I, Bezzi N, Kaci MM (2022) Valorization of olive–pomace as a green sorbent to remove Basic Red 46 (BR46) dye from aqueous solution. Biomass Convers Biorefin 46. https://doi.org/10.1007/s13399-022-03639-y
Atmani F, Kaci MM, Yeddou-Mezenner N, Soukeur A, Akkari I, Navio JA (2022) Insights into the physicochemical properties of sugar scum as a sustainable biosorbent derived from sugar refinery waste for efficient cationic dye removal. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02646-3
Tulu D, Aleme M, Mengistu G, Bogale A, Shifa K, Mendesil E (2022) Evaluation of castor (Ricinus communis L.) genotypes and their feeding values on rearing performance of eri silkworm (Samia cynthia ricini Boisduval) (Lepidoptera: Saturniidae) in Southwest Ethiopia, Psyche. J Entomol 2022. https://doi.org/10.1155/2022/1556776
Saribiyik OY, Özcanli M, Serin H, Serin S, Aydin K (2010) Biodiesel production from ricinus communis oil and its blends with soybean biodiesel, Stroj. Vestnik/Journal. Mech Eng 56:811–816. https://doi.org/10.5545/sv-jme.2009.054
Yuen FK, Hameed BH (2009) Recent developments in the preparation and regeneration of activated carbons by microwaves. Adv Colloid Interf Sci 149:19–27
Tsai WT, Chen HR (2010) Removal of malachite green from aqueous solution using low-cost chlorella-based biomass. J Hazard Mater 175:844–849. https://doi.org/10.1016/j.jhazmat.2009.10.087
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66:319–329. https://doi.org/10.1016/j.aquatox.2003.09.008
Rao KV (1995) Inhibition of DNA synthesis in primary rat hepatocyte cultures by malachite green: a new liver tumor promoter. Toxicol Lett 81:107–113. https://doi.org/10.1016/0378-4274(95)03413-7
Kumar A, Prasad B, Mishra IM (2008) Adsorptive removal of acrylonitrile by commercial grade activated carbon: kinetics, equilibrium and thermodynamics. J Hazard Mater 152:589–600. https://doi.org/10.1016/j.jhazmat.2007.07.048
Hidayat ARP, Sulistiono DO, Murwani IK, Endrawati BF, Fansuri H, Zulfa LL, Ediati R (2021) Linear and nonlinear isotherm, kinetic and thermodynamic behavior of methyl orange adsorption using modulated Al2O3@UiO-66 via acetic acid. J Environ Chem Eng 9:106675. https://doi.org/10.1016/j.jece.2021.106675
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Zheng H, Liu D, Zheng Y, Liang S, Liu Z (2009) Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J Hazard Mater 167:141–147. https://doi.org/10.1016/j.jhazmat.2008.12.093
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Li M, Liu H, Chen T, Dong C, Sun Y (2019) Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci Total Environ 651:1020–1028. https://doi.org/10.1016/J.SCITOTENV.2018.09.259
Yuan F, Li S, Fan Z, Meng X, Fan L, Yang S (2016) Shining carbon dots: synthesis and biomedical and optoelectronic applications. Nano Today 11:565–586
Dowell F, Wang D, Xu F, Yu J, Tesso T, Dowell F, Wang D (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A. Appl Energy 104:801–809. https://doi.org/10.1016/j.apenergy.2012.12.019
Brebu M, Vasile C (2010) Thermal degradation of lignin—a review. Cellul Chem Technol 44:353
Oh G-H, Yun C-H, Park C-R (2003) Role of KOH in the one-stage KOH activation of cellulosic biomass. Carbon Lett 4:180–184
Sun XF, Sun RC, Fowler P, Baird MS (2004) Isolation and characterisation of cellulose obtained by a two-stage treatment with organosolv and cyanamide activated hydrogen peroxide from wheat straw. Carbohydr Polym 55:379–391
Mu C, Jiang M, Zhu J, Zhao M, Zhu S, Zhou Z (2014) Isolation of cellulose from steam-exploded rice straw with aniline catalyzing dimethyl formamide aqueous solution. Renew Energy 63:324–329
Xie W, Peng H, Chen L (2006) Transesterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl Catal A Gen 300:67–74
Santhi T, Manonmani S, Smitha T (2010) Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of Ricinus communis by adsorption. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2010.02.076
Karatas M (2012) Removal of Pb (II) from water by natural zeolitic tuff: kinetics and thermodynamics. J Hazard Mater 199:383–389
Wei W, Wang Q, Li A, Yang J, Ma F, Pi S, Wu D (2016) Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: adsorption behavior and mechanism assessment. Sci Rep 6:1–10. https://doi.org/10.1038/srep31575
Banerjee S, Gautam RK, Jaiswal A, Gautam PK, Chattopadhyaya MC (2016) Study on adsorption behavior of Acid Orange 10 onto modified wheat husk. Desalin Water Treat 57:12302–12315. https://doi.org/10.1080/19443994.2015.1046151
Oz GAPM, Lorke DE, Hasan M (2011) Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev 31:93–117
Iscen CF, Kiran I, Ilhan S (2007) Biosorption of reactive black 5 dye by Penicillium restrictum: the kinetic study. J Hazard Mater 143:335–340. https://doi.org/10.1016/j.jhazmat.2006.09.028
Chakraborty S, Chowdhury S, Das Saha P (2011) Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym 86:1533–1541. https://doi.org/10.1016/j.carbpol.2011.06.058
Iscen SLCF, Krian I (2007) Biosorption of reactive blacks dyes by Fenicilliumrestrictum: the kinetic study. J Hazard Mater 143:335–338
Thakur A, Kaur H (2017) Response surface optimization of Rhodamine B dye removal using paper industry waste as adsorbent. Int J Ind Chem 8:175–186. https://doi.org/10.1007/s40090-017-0113-4
Dawood S, Sen TK, Phan C (2016) Adsorption removal of methylene blue (MB) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark: kinetic, equilibrium, mechanism, thermodynamic and process design. Desalin Water Treat 3994:1–17. https://doi.org/10.1080/19443994.2016.1188732
Atallah H, El M, Mahmoud A, Jelle A, Lough MH (2018) A highly stable indium based metal organic framework for efficient arsenic removal from water, Dalt. Trans 47:799–806
Cai H, Xu L, Chen G, Peng C, Ke F, Liu Z, Li D, Zhang Z, Wan X (2016) Removal of fluoride from drinking water using modified ultrafine tea powder processed using a ball-mill. Appl Surf Sci 375:74–84
Qiu Y, Zheng Z, Zhou Z, Sheng GD Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour Technol 100(2009):5348–5351. https://doi.org/10.1016/j.biortech.2009.05.054
Liu P, Liu W-J, Jiang H, Chen J-J, Li W-W, Yu H-Q (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:235–240. https://doi.org/10.1016/j.biortech.2012.06.085
Saravanan P, Thillainayagam BP, Ravindiran G, Josephraj J (2020) Evaluation of the adsorption capacity of Cocos Nucifera shell derived biochar for basic dyes sequestration from aqueous solution, Energy Sources. Part A Recover Util Environ Eff 00:1–17. https://doi.org/10.1080/15567036.2020.1800142
Jegan J, Praveen S, Pushpa TB, Gokulan R (2020) Biodecolorization of basic violet 03 using biochar derived from agricultural wastes: isotherm and kinetics. J Biobaased Mater Bioenergy 14:316–326
Munagapati VS, Wen JC, Pan CL, Gutha Y, Wen JH, Reddy GM (2020) Adsorptive removal of anionic dye (reactive black 5) from aqueous solution using chemically modified banana peel powder: kinetic, isotherm, thermodynamic, and reusability studies. Int J Phytoremediation 22:267–278. https://doi.org/10.1080/15226514.2019.1658709
Rehman R, Farooq S, Mahmud T (2019) Use of agro-waste Musa acuminata and Solanum tuberosum peels for economical sorptive removal of emerald green dye in an ecofriendly way. J Clean Prod 206:819–826. https://doi.org/10.1016/J.JCLEPRO.2018.09.226
Shakoor S, Nasar A (2017) Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using Cucumis sativus peel waste as a low-cost adsorbent, Ground. Sustain Dev 5:152–159. https://doi.org/10.1016/J.GSD.2017.06.005
Enenebeaku CK, Okorocha NJ, Enenebeaku UE, Ukaga IC (2017) Adsorption and equilibrium studies on the removal of methyl red from aqueous solution using white potato peel powder. Int Lett Chem Phys Astron 72:52–64. https://doi.org/10.18052/www.scipress.com/ILCPA.72.52
Mas Haris MRH, Sathasivam K (2009) The removal of methyl red from aqueous solutions using banana pseudostem fibers. Am J Appl Sci 6:1690–1700. https://doi.org/10.3844/ajassp.2009.1690.1700
Cavas L, Karabay Z, Alyuruk H, Doğan H, Demir GK (2011) Thomas, and artificial neural network models for the fixed-bed adsorption of methylene blue by a beach waste Posidonia oceanica (L.) dead leaves. Chem Eng J 171:557–562
Acknowledgements
The author is also thankful to the Center for the Environment and Central Instrument Facility (CIF), IIT Guwahati, for providing necessary instrumental facilities.
Funding
The financial support by DBT (Department of Biotechnology) New Delhi, India, through research grants BT/COE/34/SP28408/2018 has been highly acknowledged.
Author information
Authors and Affiliations
Contributions
Conceptualization: GD, PPB; methodology: PPB; formal analysis and investigation: PPB; writing — original draft preparation: PPB, GD; writing — review and editing: PPB, GD; funding acquisition: GD; resources: GD, PPB; supervision: GD.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3928 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakal, P.P., Das, G. A Comparative Study of Raw vs. Activated Biochar Derived from “Ricinus communis Stem” for Preferential Removal of Cationic Dyes. Water Conserv Sci Eng 8, 19 (2023). https://doi.org/10.1007/s41101-023-00192-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41101-023-00192-1