Abstract
Owing to the high energy and power density of lithium-ion cells (1200 Wh kg−1 and 200 Wh kg−1) and due to their compact design, they are used as energy storage devices in many contemporary mobile applications such as telecommunication systems, notebooks and domestic appliances. Meanwhile their application is not limited only to consumer electronics, they are also standard in hybrid electric (HEVs) and electric vehicles (EVs). However, the profitable application of lithium-ion cells in the automobile industry requires lower costs, lower safety risks, a higher specific energy density and a longer lifetime under everyday conditions. All these aspects are directly or indirectly related to the degradation of the materials in a lithium-ion cell. One possibility for reducing the costs is a second life application of the cells after their usage in (H)EVs. In order to enable this, the safety risks at the end of life of a cell operated in a vehicle have to be reliably predicted. This requires a fundamental knowledge about underlying material degradations during operation. The safety risk of a lithium-ion cell increases during operation because the voltage windows in which the electrodes are cycled shift, resulting in a higher possibility that at least one electrode is operated in a meta- or unstable state. Furthermore, higher impedances due to material degradations lead to increasing heat generation and therefore to an increase in the risk of failure. Higher energy densities can be achieved by raising the end of charge voltage of a cell, causing additional safety risks because many cathode materials tend to decompose at high voltages. Another possibility for achieving higher energy densities is to use nickel-rich or lithium-excess cathode materials, since cathodes are currently limiting the capacity of lithium-ion cells. But these systems show a poor cycling stability (a higher degradation rate). The lifetime of a lithium-ion cell is limited by the degradation of the individual cell components. Although the degradation of materials is the key consideration in achieving lower costs, a higher safety standard, higher energy densities and a longer lifetime, the degradation of the individual cell components in dependence on the operation conditions has hardly been investigated and is poorly understood. The present work reviews known material degradations in commercial lithium-ion cells, shows a way to analyze such degradations in dependence on the operation conditions and describes how these degradation processes lead to observed performance drops.































Similar content being viewed by others
References
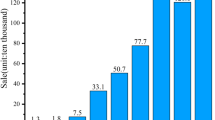
Pillot C (2013) Li-ion battery material market review and forecasts 2012–2025. In: Avicenne energy, 3rd israeli power sources conference, May 29–30, 2013
Andre D, Kim S-J, Lamp P, Lux SF, Maglia F, Paschos O, Stiaszny B (2015) Future generations of cathode materials: an automotive industry perspective. J Mater Chem A 3:6709–6732
Kleiner K (2014) Chemical investigation of aging mechanisms in lithium-ion batteries; PhD thesis, Cuvilliier Verlag Göttingen
Broussely M, Biensan P, Bonhomme F, Blanchard P, Herreyre S, Nechev K, Staniewicz RJJ (2005) Main aging mechanisms in Li Ion batteries. J Power Sources 146(1–2):90–96
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603
Aurbach D, Zinigrad E, Cohen Y, Teller H (2002) A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid state ion 148(3–4):405–416
Wohlfahrt-Mehrens M, Vogler C, Garche J (2004) Aging mechanisms of lithium cathode materials. J Power Sources 127:58–64
Vetter J, Novák P, Wagner MR, Veit C, Möller K-C, Besenhard JO, Winter M, Wohlfahrt-Mehrens M, Vogler C, Hammouche A (2005) Ageing mechanisms in lithium-ion batteries. J Power Sources 147(1–2):269–281
Arora P, White RE, Doyle M (1998) Capacity fade mechanisms and side reactions in lithium-ion batteries. J Electrochem Soc 145(10):3647–3667
Peled E (1979) The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J Electrochem Soc 126(12):2047–2051
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4417
Peled E, Bar Tow D, Merson A, Gladkich A, Burstein L, Golodnitsky D (2001) Composition, Depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies. J Power Sources 98:52–57
Aurbach D, Levi MD, Levi E, Schechter A (1997) Failure and stabilization mechanisms of graphite electrodes. J Phys Chem B 101(12):2195–2206
Aurbach D, Gofer Y, Ben-Zion M, Aped P (1992) The behaviour of lithium electrodes in propylene and ethylene carbonate: Te major factors that influence Li cycling efficiency. J Electroanal Chem 339(1–2):451–471
Nazri G-A, Pistoia G (eds) (2003) Lithium batteries-science and technology. Springer, New York
Kleiner K, Melke J, Merz M, Jakes P, Nagel P, Schuppler S, Liebau V, Ehrenberg H (2015) Unraveling the degradation process of LiNi 0.8 Co 0.15 Al 0.05 O2 electrodes in commercial lithium ion batteries by electronic structure investigations. ACS Appl Mater Interfaces 7:19589–19600
Kojima Y, Muto S, Tatsumi K, Kondo H, Oka H, Horibuchi K, Ukyo Y (2011) Degradation analysis of a Ni-based layered positive-electrode active material cycled at elevated temperatures studied by scanning transmission electron microscopy and electron energy-loss spectroscopy. J Power Sources 196(18):7721–7727
Hausbrand R, Cherkashinin G, Ehrenberg H, Gröting M, Albe K, Hess C, Jaegermann W (2015) Fundamental degradation mechanisms of layered oxide Li-Ion battery cathode materials: methodology, insights and novel approaches. Mater Sci Eng B 192:3–25
Laubach S, Laubach S, Schmidt PC, Ensling D, Schmid S, Jaegermann W, Thissen A, Nikolowski K, Ehrenberg H (2009) Changes in the crystal and electronic structure of LiCoO(2) and LiNiO(2) upon Li intercalation and de-intercalation. Phys Chem Chem Phys 11:3278–3289
Aoshima T, Okahara K, Kiyohara C, Shizuka K (2001) Mechanisms of manganese spinels dissolution and capacity fade at high temperature. J Power Sources 97–98:377–380
Kleiner K, Jackes P, Liebau V, Scharner S, Ehrenberg H (2016) Changes of the balancing between anode and cathode due to fatigue in commercial lithium-ion cells. J Power Sources 317:25–34
Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224
Hoseh M (1960) Some problems in chemical kinetics and reactivity, vol 1. Princeton University Press, Princeton
Xu K, Lam Y, Zhang SS, Jow TR, Curtis TB (2007) Solvation sheath of Li+ in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J Phys Chem C 111(20):7411–7421
Xu K (2009) Whether EC and PC differ in interphasial chemistry on graphitic anode and how. J Electrochem Soc 156(9):A751
Aurbach D, Daroux ML, Faguy PW, Yeager E (1987) Identification of surface films formed on lithium in propylene carbonate solutions. J Electrochem Soc 134(7):1611–1620
Augustsson A, Herstedt M, Guo J-H, Edström K, Zhunag GV, Ross PN, Rubensson J-E, Nordgren J (2004) Solid electrolyte interphase on graphite Li-Ion battery anodes studied by soft X-ray spectroscopy. Phys Chem Chem Phys 6(16):4185–4189
Malmgren S, Rensmo H, Gustafsson T, Gorgoi M, Edström K (2010) Nondestructive depth profiling of the solid electrolyte interphase on LiFePO4. ECS Trans 25(36):201–210
Li Z, Huang J, Yann Liaw B, Metzler V, Zhang J (2014) A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J Power Sources 254:168–182
Agubra V, Fergus J (2013) Lithium ion battery anode aging mechanisms. Materials (Basel). 6(4):1310–1325
Koltypin M, Cohen YS, Markovsky B, Cohen Y, Aurbach D (2002) The study of lithium insertion-deinsertion processes into composite graphite electrodes by in situ atomic force microscopy (AFM). Electrochem Commun 4:17–23
Wagner MR, Albering JH, Moeller K-C, Besenhard JO, Winter M (2005) XRD evidence for the electrochemical formation of Li+ (PC)yCn− in PC-based electrolytes. Electrochem Commun 7(9):947–952
Aurbach D (1993) In situ FTIR spectroelectrochemical studies of surface films formed on Li and nonactive electrodes at low potentials in Li salt solutions containing CO[sub 2]. J Electrochem Soc 140(11):L155
Broussely M, Herreyre S, Biensan P, Kasztejna P, Nechev K, Staniewicz R (2001) Aging mechanism in Li Ion cells and calendar life predictions. J Power Sources 97–98:13–21
Verma P, Maire P, Novák P (2010) A review of the features and analyses of the solid electrolyte interphase in Li-Ion batteries. Electrochim Acta 55(22):6332–6341
Yazami R, Reynier Y (2002) Mechanism of self-discharge in graphite–lithium anode. Electrochim Acta 47(8):1217–1223
Utsunomiya T, Hatozaki O, Yoshimoto N, Egashira M, Morita M (2011) Self-discharge behavior and its temperature dependence of carbon electrodes in lithium-ion batteries. J Power Sources 196(20):8598–8603
Zhu Y, Wang C (2010) Galvanostatic intermittent titration technique for phase-transformation electrodes. J Phys Chem C 114(6):2830–2841
Sloop SE, Kerr JB, Kinoshita K (2003) The role of Li-Ion battery electrolyte reactivity in performance decline and self-discharge. J Power Sources 119–121:330–337
Sugiyama J, Mukai K, Ikedo Y, Nozaki H, Russo PL, Andreica D, Amato A, Ariyoshi K, Ohzuku T (2009) Static magnetic order on the triangular lattice in with. Phys B Condens Matter 404(5–7):663–666
Wang Y, Nakamura S, Tasaki K, Balbuena PB (2002) Theoretical studies to understand surface chemistry on carbon anodes for lithium-ion batteries: how does vinylene carbonate play its role as an electrolyte additive? J Am Chem Soc 124(16):4408–4421
Aurbach D (2003) Electrode–solution interactions in Li-ion batteries: a short summary and new insights. J Power Sources 119–121:497–503
Steiger J, Richter G, Wenk M, Kramer D, Mönig R (2015) Comparison of the growth of lithium filaments and dendrites under different conditions. Electrochem Commun 50:11–14
Orsini F, du Pasquier A, Beaudouin B, Tarascon JM, Trentin M, Langenhuizen N, de Beer E, Notten P (1999) In situ SEM study of the interfaces in plastic lithium cells. J Power Sources 81–82:918–921
Brissot C (1999) In situ concentration cartography in the neighborhood of dendrites growing in lithium/polymer-electrolyte/lithium cells. J Electrochem Soc 146(12):4393
Kleiner K, Comas-Vives A, Naderian M, Mueller JE, Fantauzzi D, Mesgar M, Keith JA, Anton J, Jacob T (2011) Multiscale modeling of Au-Island ripening on Au(100). Adv Phys Chem 2011:1–11
Steiger J, Kramer D, Mönig R (2014) Mechanisms of dendritic growth investigated by in situ light microscopy during electrodeposition and dissolution of lithium. J Power Sources 261:112–119
Steiger J, Kramer D, Mönig R (2014) Microscopic observations of the formation, growth and shrinkage of lithium moss during electrodeposition and dissolution. Electrochim Acta 136:529–536
Morigaki K, Ohta A (1998) Analysis of the surface of lithium in organic electrolyte by atomic force microscopy, fourier transform infrared spectroscopy and scanning auger electron microscopy. J Power Sources 76:159–166
Diggle JW, Despic AR, Bockris JO (1969) The mechanism of the dendritic electrocrystallization of zinc. J Electrochem Soc 116(11):1503
Yamaki J, Tobishima S, Hayashi K, Saito K, Nemoto Y, Arakawa MA (1998) Consideration of the morphology of electrochemically deposited lithium in an organic electrolyte. J Power Sources 74:219–227
Yamaki J-I, Tobishima S-I (2011) Rechargeable lithium anodes. In: Besenhard JO (ed) Handbook of battery materials, 2nd edn. Wiley-VCH Verlag GmbH & Co, Weinheim
Kim C-S, Jeong KM, Kim K, Yi C-W (2015) Effects of capacity ratios between anode and cathode on electrochemical properties for lithium polymer batteries. Electrochim Acta 155:431–436
Purushothaman BK, Landau U (2006) Rapid charging of lithium-ion batteries using pulsed currents a theoretical analysis. J Electrochem Soc 153:A533–A542
Jow RT, Xu K, Borodin O, Ue M (2014) Electrolytes for lithium and lithium-ion batteries, modern Asp. Springer, New York
Krueger S, Kloepsch R, Li J, Nowak S, Passerini S, Winter M (2013) How do reactions at the anode/electrolyte interface determine the cathode performance in lithium-ion batteries? J Electrochem Soc 160(4):A542–A548
Kawamura T, Okada S, Yamaki J (2006) Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J Power Sources 156(2):547–554
Sloop SE, Pugh JK, Wang S, Kerr JB, Kinoshita K (2001) Chemical reactivity of PF[sub 5] and LiPF[sub 6] in ethylene carbonate/dimethyl carbonate solutions. Electrochem Solid State Lett 4(4):A42
Campion CL, Li W, Lucht BL (2005) Thermal decomposition of LiPF[sub 6]-based electrolytes for lithium-ion batteries. J Electrochem Soc 152(12):A2327
Zhang SS (2007) A review on the separators of liquid electrolyte li-ion batteries. J Power Sources 164:351–364
Baginska M, Blaiszik BJ, Merriman RJ, Sottos NR, Moore JS, White SR (2012) Autonomic shutdown of lithium-ion batteries using thermoresponsive microspheres. Adv Energy Mater 2:583–590
Love CT (2011) Thermomechanical analysis and durability of commercial micro-porous polymer Li-Ion battery separators. J Power Sources 196(5):2905–2912
Norin L, Kostecki R, McLarnon F (2002) Study of membrane degradation in high-power lithium-ion cells. Electrochem Solid State Lett 5:A67
Gallus DR, Schmitz R, Wagner R, Hoffmann B, Nowak S, Cekic-Laskovic I, Schmitz RW, Winter M (2014) The influence of different conducting salts on the metal dissolution and capacity fading of NCM cathode material. Electrochim Acta 134:393–398
Zheng H, Sun Q, Liu G, Song X, Battaglia VS (2012) Correlation between dissolution behavior and electrochemical cycling performance for LiNi 1/3 Co 1/3 Mn 1/3 O2-based cells. J Power Sources 207:134–140
Hunter JC (1981) Preparation of a new crystal form of manganese dioxide: λ-MnO2. J Solid State Chem 39:142–147
Jang DH, Shin YJ, Oh SM (1996) Dissolution of spinel oxides and capacity losses in 4 V. J Electrochem Soc 143(7):2204–2211
Aurbach D, Levi MD, Gamulski K, Markovsky B, Salitra G, Levi E, Heider U, Heider L, Oesten R (1999) Capacity fading of Li x Mn2O4 spinel electrodes studied by XRD and electroanalytical techniques. J Power Sources 81–82:472–479
Yang K, Fan L-Z, Guo J, Qu X (2012) Significant improvement of electrochemical properties of AlF3-coated LiNi0.5Co0.2Mn0.3O2 cathode materials. Electrochim Acta 63:363–368
Li GR, Feng X, Ding Y, Ye SH, Gao XP (2012) AlF3-coated Li(Li0.17Ni0.25Mn0.58)O2 as cathode material for Li-Ion batteries. Electrochim Acta 78:308–315
Lee SH, Koo BK, Kim J-C, Kim KM (2008) Effect of Co3(PO4)2 coating on Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathode material for lithium rechargeable batteries. J Power Sources 184:276–283
Chen Z, Qin Y, Amine K, Sun Y-K (2010) Role of surface coating on cathode materials for lithium-ion batteries. J Mater Chem 20:7606
Martha SK, Sclar H, Szmuk Framowitz Z, Kovacheva D, Saliyski N, Gofer Y, Sharon P, Golik E, Markovsky B, Aurbach D (2009) A comparative study of electrodes comprising nanometric and submicron particles of LiNi0.50Mn0.50O2, LiNi0.33Mn0.33Co0.33O2, and LiNi0.40Mn0.40Co0.20O2 layered compounds. J Power Sources 189:248–255
Jansen A, Kahaian A, Kepler K, Nelson P, Amine K, Dees D, Vissers D, Thackeray M (1999) Development of a high-power lithium-ion battery. J Power Sources 81–82:902–905
Li J, Lewis RB, Dahn JR (2007) Sodium carboxymethyl cellulose. Electrochem Solid State Lett 10(2):A17
Zhang WJ (2011) A Review of the electrochemical performance of alloy anodes for lithium-ion batteries. J Power Sources 196(1):13–24
Buqa H, Holzapfel M, Krumeich F, Veit C, Novák P (2006) Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J Power Sources 161:617–622
Hochgatterer NS, Schweiger MR, Koller S, Raimann PR, Wöhrle T, Wurm C, Winter M (2008) Silicon/graphite composite electrodes for high-capacity anodes: influence of binder chemistry on cycling stability. Electrochem Solid-State Lett 11(5):A76
Xu J, Chou S-L, Gu Q, Liu H-K, Dou S-X (2013) The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J Power Sources 225:172–178
Manickam M, Takata M (2003) Effect of cathode binder on capacity retention and cycle life in transition metal phosphate of a rechargeable lithium battery. Electrochim Acta 48(8):957–963
Chen Z, Chevrier V, Christensen L, Dahn JR (2004) Design of amorphous alloy electrodes for Li-Ion batteries. Electrochem Solid State Lett 7(10):A310
Liu W-R, Yang M-H, Wu H-C, Chiao SM, Wu N-L (2005) Enhanced cycle life of Si anode for Li-Ion batteries by using modified elastomeric binder. Electrochem Solid State Lett 8(2):A100
Markevich E, Salitra G, Aurbach D (2005) Influence of the PVdF binder on the stability of LiCoO2 electrodes. Electrochem Commun 7(12):1298–1304
Aurbach D, Markovsky B, Salitra G, Markevich E, Talyossef Y, Koltypin M, Nazar L, Ellis B, Kovacheva D (2007) Review on electrode–electrolyte solution interactions, related to cathode materials for Li-Ion batteries. J Power Sources 165:491–499
Lee J-H, Paik U, Hackley VA, Choi Y-M (2005) Effect of carboxymethyl cellulose on aqueous processing of natural graphite negative electrodes and their electrochemical performance for lithium batteries. J Electrochem Soc 152(9):A1763
Choi Y-M, Paik U, Kim K-H (2003) Application No. P2003-0040085
Spotnitz R, Franklin J (2003) Abuse behavior of high-power, lithium-ion cells. J Power Sources 113:81–100
Li G (1996) The influence of polytetrafluorethylene reduction on the capacity loss of the carbon anode for lithium ion batteries. Solid State Ion 90:221–225
Maleki H, Deng G, Kerzhner-Haller I (2000) Thermal stability studies of binder materials in anodes for lithium ion batteries. J Electrochem Soc 147(12):4470–4475
Buqa H, Goers D, Spahr ME, Novak P (2003) The influence of graphite surface modification on the exfoliation during electrochemical lithium insertion. J Solid State Electrochem 8(1):79–80
Zackrisson M, Avellán L, Orlenius J (2010) Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles-critical issues. J Clean Prod 18(15):1517–1527
Kim GT, Jeong SS, Joost M, Rocca E, Winter M, Passerini S, Balducci A (2011) Use of natural binders and ionic liquid electrolytes for greener and safer lithium-ion batteries. J Power Sources 196(4):2187–2194
Saeki S, Lee J, Zhang Q, Saito F (2004) Co-Grinding LiCoO2 with PVC and water leaching of metal chlorides formed in ground product. Int J Miner Process 74:S373–S378
Li C-C, Lee J-T, Tung Y-L, Yang C-R (2007) Effects of pH on the dispersion and cell performance of LiCoO2 cathodes based on the aqueous process. J Mater Sci 42(14):5773–5777
Chou S-L, Pan Y, Wang J-Z, Liu H-K, Dou S-X (2014) Small things make a big difference: binder effects on the performance of Li and Na batteries. Phys Chem Chem Phys 16(38):20347–20359
Guerfi A, Kaneko M, Petitclerc M, Mori M, Zaghib K (2007) LiFePO4 water-soluble binder electrode for li-ion batteries. J Power Sources 163(2):1047–1052
Porcher W, Lestriez B, Jouanneau S, Guyomard D (2009) Design of aqueous processed thick LiFePO[sub 4] composite electrodes for high-energy lithium battery. J Electrochem Soc 156(3):A133
Li J, Murphy E, Winnick J, Kohl PA (2001) Studies on the cycle life of commercial lithium ion batteries during rapid charge-discharge cycling. J Power Sources 102:294–301
Chen D, Indris S, Schulz M, Gamer B, Mönig R (2011) In situ scanning electron microscopy on lithium-ion battery electrodes using an ionic liquid. J Power Sources 196(15):6382–6387
Kızıltaş-Yavuz N, Herklotz M, Hashem AM, Abuzeid HM, Schwarz B, Ehrenberg H, Mauger A, Julien CM (2013) Synthesis, structural, magnetic and electrochemical properties of LiNi1/3Mn1/3Co1/3O2 Prepared by a sol–gel method using table sugar as chelating agent. Electrochim Acta 113:313–321
Park M, Zhang X, Chung M, Less GB, Sastry AM (2010) A review of conduction phenomena in Li-Ion batteries. J Power Sources 195(24):7904–7929
Amin R, Ravnsbæk DB, Chiang Y-M (2015) Characterization of electronic and ionic transport in Li1−x Ni0.8Co0.15Al0.05O2 (NCA). J Electrochem Soc 162(7):A1163–A1169
Nobili F, Croce F, Scrosati B, Marassi R (2001) Electronic and electrochemical properties of Li x Ni1−y Co y O2 cathodes studied by impedance spectroscopy. Chem Mater 13:1642–1646
Kleiner K, Dixon D, Jakes P, Melke J, Murat Y, Roth C, Nikolowski K, Liebau V, Ehrenberg H (2015) Fatigue of LiNi0.8Co0.15Al0.05O2 in commercial Li ion batteries. J Power Sources 273:70–82
Xiao J, Yu X, Zheng J, Zhou Y, Gao F, Chen X, Bai J, Yang XQ, Zhang JG (2013) Interplay between two-phase and solid solution reactions in high voltage spinel cathode material for lithium ion batteries. J Power Sources 242:736–741
Senyshyn A, Mühlbauer MJ, Nikolowski K, Pirling T, Ehrenberg H (2012) “In-operando” neutron scattering studies on Li-Ion batteries. J Power Sources 203:126–129
Padhi A, Nanjundaswamya K, Goodenough J (1997) Phospho-Olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Brunetti G, Robert D, Bayle-Guillemaud P, Rouvière JL, Rauch EF, Martin JF, Colin JF, Bertin F, Cayron C (2011) Confirmation of the Domino-Cascade model by lifepo4/fepo 4 precession electron diffraction. Chem Mater 23:4515–4524
Manthiram A, Sivaramakrishnan V (2001) Chemical and structural instabilities of LiCoO2 cathodes at deep lithium extraction. Materials science and engineering program, ETC 9.104, Austin
Arunkumar TA, Alvarez E, Manthiram A (2008) Chemical and structural instability of the chemically delithiated (1−Z) Li Li1/3Mn2/3 O-2 center dot(z) Li Co1−y Ni y O-2 (0 ≤ Y ≤ 1 and 0 ≤ Z ≤ 1) Solid Solution Cathodes. J Mater Chem 18:190–198
Reimers JN, Dahn JR (1992) Electrochemical and in situ X-ray diffraction studies of lithium intercalation in Li x CoO2. J Electroanal Chem 139(8):2–8
Ohzuku T, Ueda A, Nagayama M (1993) electrochemistry and structural chemistry of LiNi02 (R3 m) for 4 volt secondary lithium cells. 140(7):1862–1870
Ohzuku T, Ueda A, Kouguchi M (1995) Synthesis and characterization of LiAl1/4Ni3/4O2 (R-3m) for lithium-ion (shuttlecock) batteries. J Electrochem Soc 142(12):4033–4039
Choi S, Manthiram A (2002) Factors influencing the layered to spinel-like phase transition in layered oxide cathodes. J Electrochem Soc 149:A1157
Kang S (2002) Layered Li(Ni0.5−x Mn0.5−x M2x′)O2 (M′ = Co, Al, Ti; x = 0, 0.025) cathode materials for Li-Ion rechargeable batteries. J Power Sources 112(1):41–48
Jiang M, Key B, Meng YS, Grey CP (2009) Electrochemical and structural study of the layered, “Li-excess” lithium-ion battery electrode material Li[Li1/9Ni1/3Mn5/9]O2. Chem Mater 21(13):2733–2745
Hong J, Gwon H, Jung S-K, Ku K, Kang K (2015) Review—lithium-excess layered cathodes for lithium rechargeable batteries. J Electrochem Soc 162(14):A2447–A2467
Chebiam RV, Prado F, Manthiram A (2001) Soft chemistry synthesis and characterization of layered Li1−x Ni1−Y Co Y O2−δ (0 <= x <= 1 and 0 <= y <= 1). Chem Mater 5:2951–2957
Yoon W-S, Chung KY, McBreen J, Fischer DA, Yang X-Q (2007) electronic structural changes of the electrochemically Li-Ion Deintercalated LiNi0.8Co0.15Al0.05O2 cathode material investigated by X-ray absorption spectroscopy. J Power Sources 174(2):1015–1020
Montoro L, Abbate M, Almeida E, Rosolen J (1999) Electronic structure of the transition metal ions in LiCoO2, LiNiO2 and LiCo0.5Ni0.5O2. Chem Phys Lett 309(1–2):14–18
Senyshyn A, Dolotko O, Muhlbauer MJ, Nikolowski K, Fuess H, Ehrenberg H (2013) Lithium intercalation into graphitic carbons revisited: experimental evidence for twisted bilayer behavior. J Electrochem Soc 160(5):A3198–A3205
Levi MD, Wang C, Markevich E, Aurbach D, Chvoj Z (2003) Noteworthy electroanalytical features of the stage 4 to stage 3 phase transition in lithiated graphite. J Solid State Electrochem 8(1):40–43
Ohzuku T, Kitagawa M, Hitai T (1990) Electrochemistry of manganese dioxide in lithium nonaqueous Cell II. X-ray diffractional and electrochemical characterization on deep discharge products of electrolytic manganese dioxide. J Electrochem Soc 137(3):40–46
Shao-horn Y, Hackney SA, Kahaian AJ, Kepler KD, Skinner E, Vaughey JT, Thackeray MM (1999) Structural fatigue in spinel electrodes in Li R Li X W Mn 2 X O 4 cells. J Power Sources 81–82:496–499
Aurbach D, Levi M, Gamulski K, Markovsky B, Salitra G, Levi E, Heider U, Heider L, Oesten R (1999) Capacity fading of Li x Mn2O4 spinel electrodes studied by XRD and electroanalytical techniques. J Power Sources 81–82:472–479
Chung KY, Kim KB (2004) Investigations into capacity fading as a result of a Jahn-Teller distortion in 4 V LiMn2O4 thin film electrodes. Electrochim Acta 49:3327–3337
Ikeno H, de Groot FMF, Stavitski E, Tanaka I (2009) Multiplet calculations of L(2,3) X-Ray absorption near-edge structures for 3d transition-metal compounds. J Phys Condens Matter 21(10):104208
Tran N, Croguennec L, Menetrier M, Weill F, Biensan P, Jordy C, Delmas C (2008) Mechanisms associated with the “Plateau” observed at high voltage for the overlithiated Li-1.12(Ni0.425Mn0.425Co0.15)(0.88)O-2 system. Chem Mater 20(6):4815–4825
Wohlfahrt-Mehrens M, Butz A, Oesten R, Arnold G, Hemmer RP, Huggins RA (1997) The influence of doping on the operation of lithium manganese oxide spinel. J Power Sources 68(2):582–585
Guao Y, Dahn JR (1996) Synthesis and characterization of Li1 + Mn2O4 for Li-Ion battery applications. J Electrochem Soc 143(1):100–114
Rozier P, Tarascon JM (2015) Review—Li-Rich layered oxide cathodes for next-generation Li-Ion batteries: chances and challenges. J Electrochem Soc 162(14):A2490–A2499
Montoro LA, Rosolen JM (2004) The role of structural and electronic alterations on the lithium diffusion in Li x Co0.5Ni0.5O2. Electrochim Acta 49(19):3243–3249
Yoon W, Kim K, Kim M, Lee M, Shin H, Lee J, Lee J (2002) Oxygen contribution on Li-Ion intercalation—deintercalation in LiCoO2 investigated by O K-edge and Co L-edge X-ray absorption spectroscopy. J Phys Chem B 106:2526–2532
Kobayashi H, Arachi Y, Emura S, Tatsumi K (2007) Investigation on lithium de-intercalation mechanism for LiNi0.45Mn0.45Al0.1O2. Solid State Ion 178:1101–1105
Van der Ven A, Ceder G (2000) Lithium diffusion mechanisms in layered intercalation compounds. J Power Sources 97–98:529–531
Moses AW, Flores HGG, Kim J-G, Langell MA (2007) Surface properties of LiCoO2, LiNiO2 and LiNi1−x Co x O2. Appl Surf Sci 253:4782–4791
Han CJ, Yoon JH, Cho WL, Jang H (2004) Electrochemical properties of LiNi0.8Co0.2−x Al x O2 prepared by a sol–gel method. J Power Sources 136(1):132–138
Yang XQ, Sun X, McBreen J (1999) New findings on the phase transitions in Li1−x NiO2: in situ synchrotron X-ray diffraction studies. Electrochem Commun 1(6):227–232
Saito Y, Shikano M, Kobayashi H (2011) State of charge (SOC) dependence of lithium carbonate on LiNi0.8Co0.15Al0.05O2 electrode for lithium-ion batteries. J Power Sources 196(16):6889–6892
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst A32:751–767
Maier J (ed) (2000) Festkörper-Fehler Und Funktion. Teubner Studienbücher, Leipzig
Amalraj F, Talianker M, Markovsky B, Sharon D, Burlaka L, Shafir G, Zinigrad E, Haik O, Aurbach D, Lampert J, Schulz-Dobrick M, Garsuch A (2012) Study of the lithium-rich integrated compound x Li2MnO3(1−x)LiMO2 (X around 0.5; M = Mn, Ni, Co; 2:2:1) and its electrochemical activity as positive electrode in lithium cells. J Electrochem Soc 160(2):A324–A337
Chanson C, Wiaux J-P (2013) Safety of lithium-ion batteries. The European Association for advanced rechargeable batteries. Belgium, Brussels, p 25
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Electrochemical Energy Storage”, edited by Rüdiger A. Eichel.
Rights and permissions
About this article
Cite this article
Kleiner, K., Ehrenberg, H. Challenges Considering the Degradation of Cell Components in Commercial Lithium-Ion Cells: A Review and Evaluation of Present Systems. Top Curr Chem (Z) 375, 54 (2017). https://doi.org/10.1007/s41061-017-0139-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-017-0139-2