Highlights
-
The 1T-WS2@TiO2@Ti3C2 photocatalyst is highly active for water splitting to produce hydrogen at 3409.8 μmol g−1 h−1.
-
The Ti3C2 MXene and octahedral (1T) phase WS2 act pathways transferring photogenerated electrons.
Abstract
The biggest challenging issue in photocatalysis is efficient separation of the photoinduced carriers and the aggregation of photoexcited electrons on photocatalyst’s surface. In this paper, we report that double metallic co-catalysts Ti3C2 MXene and metallic octahedral (1T) phase tungsten disulfide (WS2) act pathways transferring photoexcited electrons in assisting the photocatalytic H2 evolution. TiO2 nanosheets were in situ grown on highly conductive Ti3C2 MXenes and 1T-WS2 nanoparticles were then uniformly distributed on TiO2@Ti3C2 composite. Thus, a distinctive 1T-WS2@TiO2@Ti3C2 composite with double metallic co-catalysts was achieved, and the content of 1T phase reaches 73%. The photocatalytic H2 evolution performance of 1T-WS2@TiO2@Ti3C2 composite with an optimized 15 wt% WS2 ratio is nearly 50 times higher than that of TiO2 nanosheets because of conductive Ti3C2 MXene and 1T-WS2 resulting in the increase of electron transfer efficiency. Besides, the 1T-WS2 on the surface of TiO2@Ti3C2 composite enhances the Brunauer–Emmett–Teller surface area and boosts the density of active site.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to energy consumption and consequent environmental pollution, the generation of hydrogen (H2) from water using solar light through semiconductors materials has aroused great attention [1,2,3,4]. Among these, TiO2 is widely studied owing to nontoxicity and low cost [5, 6]. However, the fast photoexcited carrier recombination restricts the TiO2’s application, and thus numerous efforts, such as doping, co-catalyst loading and heterostructure designing, are made to improve photoexcited carrier separation [7, 8]. Among these, co-catalysts can gather carriers to improve separation and act as active sites for H2 production [9]. Noble metals as excellent co-catalysts have widely applied to photocatalysis. However, extreme scarcity and high price restrict their application of photocatalytic water splitting [10, 11]. Therefore, seeking an inexpensive and highly active co-catalyst is of paramount significance for achieving photocatalytic H2 production in the future [12].
MXenes, as new 2D materials, have aroused remarkable attention because of its excellent electrical conductivity [13, 14]. For example, a 2D material with an accordion-like structure of layered Ti3C2 MXene can be prepared by etching Al layers from Ti3AlC2, in HF solution [15,16,17]. Due to its high electrical conductivity and unique layer morphology, Ti3C2 MXene is an appropriate substitute co-catalyst for noble metals for photocatalytic H2 evolution [18].
In recent years, transition metal disulfides (TMDs), such as molybdenum disulfide (MoS2) and tungsten disulfide (WS2), are regarded as promising substitutes for noble metals on catalysis [19, 20]. MoS2 and WS2 mainly include semiconductive trigonal (2H) phase and metallic octahedral (1T) phase [21, 22]. Both experimental and theoretical research have revealed that the metallic 1T phase possesses outstanding conductivity and more active sites, which will be suitable co-catalyst for photocatalytic H2 evolution, compared with 2H phase [23]. As one of the most popular TMDs materials, 1T phase MoS2 has been widely studied on photocatalysis [24,25,26]. However, the report about 1T phase WS2 (1T-WS2) on photocatalytic H2 production is still rare.
In this paper, an innovative 2D heterojunction by utilizing the metallic feature of Ti3C2 MXene and 1T-WS2 is reported. A two-step hydrothermal method is used for designing the novel 1T-WS2@TiO2@Ti3C2 photocatalyst where Ti3C2 MXene and 1TWS2 play important roles as electron acceptors. Firstly, TiO2 nanosheets are in situ grown on the surface of highly conductive Ti3C2 MXenes to construct TiO2@Ti3C2 composites by a facile hydrothermal method. Secondly, we intentionally employ the 1T-WS2 nanoparticles evenly distribute on TiO2@Ti3C2 composites’ surface using a hydrothermal process. This procedure results in the construction of an efficient photocatalytic system with intimate contact among metallic Ti3C2 MXene, 1T-WS2 nanoparticles, and TiO2 NSs. The newly designed 1T-WS2@TiO2@Ti3C2 composites exhibit extremely enhanced photocatalytic H2 evolution activity and stability owing to the novel structure.
2 Experimental Procedures
2.1 Materials
Ti3AlC2 powder was purchased from 11 Technology. Hydrochloric acid (HCl), sodium tetrafluoroborate (NaBF4), hydrofluoric acid (HF, 40 wt%), tungsten chloride (WCl6), thioacetamide (TAA), and dimethylformamide (DMF) were provided by Sinopharm.
2.2 Synthesis of Ti3C2 MXenes
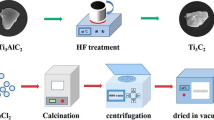
In a typical synthesis, 1 g Ti3AlC2 powders were dissolved in 120 mL HF solution (40 wt%) and were stirred for 72 h. Then, the mixed solution was washed with deionized (DI) water to neutral. Lastly, Ti3C2 MXenes were dried at 50 °C for overnight in a vacuum oven.
2.3 Synthesis of TiO2@Ti3C2 Composites
Ti3C2 MXenes (400 mg) and NaBF4 (660 mg) were dissolved in 60 mL HCl (1.0 M) and were stirred for 30 min. The mixed solution was hydrothermally treated at 160 °C for 12 h. The obtained TiO2@Ti3C2 composites were washed with DI water and dried at 60 °C for overnight in a vacuum oven.
2.4 Synthesis of 1T-WS2@TiO2@Ti3C2 Composites
WCl6 (24 mg) and TAA (9 mg) were added into 50 mL DMF. Then, 100 mg TiO2@Ti3C2 composites were dispersed in above solution and were stirred for 60 min. The mixed solution was hydrothermally treated at 200 °C for 24 h. The obtained 1T-WS2@TiO2@Ti3C2 composites (15 wt% WS2) were washed with DI water and dried at 60 °C for overnight in a vacuum oven. By adjusting the adding amount of WCl6 (16, 32, and 40 mg) and TAA (6, 12, and 15 mg), 1T-WS2@TiO2@Ti3C2 composites with other WS2 adding amounts (10, 20, and 25 wt%) were prepared, respectively.
2.5 Characterizations
The phases of the samples were carried out using D/Max 2500PC X-ray diffraction (XRD). The surface characteristic and structure of the samples were tested by a FEI Nano 450 high-resolution scanning electron microscope (FESEM) and a JEOL 2100F high transmission electron microscope (HRTEM). The chemical states of the products were analyzed by a Thermo ESCALAB 250XI X-ray photoelectron spectrometry (XPS). The specific surface area and pore size distribution were tested by a nitrogen adsorption–desorption apparatus (Micromeritics ASAP2020) using the Brunauer–Emmett–Teller (BET) method. The UV–Vis diffuse reflectance spectra (DRS) of the products were measured using a Hitachi UH3101 UV–Vis spectrophotometer. The photoluminescence (PL) spectra were tested by a FLS920 fluorescence.
2.6 Photoelectrochemical and Photocatalytic Activity Test
The photocatalytic activity test was measured using a Pyrex glass vessel, with a 300 W Xe arc lamp (CELHXF300) with an AM-1.5 filter as the light source. 10 mg of catalysts were added into acetone/TEOA solution (15 mL acetone + 5 mL TEOA + 80 mL DI water). The amount of generated H2 was tested by a gas chromatograph (Techcomp GC-7920). The electrochemical impedance spectroscopy (EIS) and transient photocurrent response (PEC) of the catalysts were measured by an electrochemical workstation (CHI660D) under a 300 W Xe arc lamp with an AM-1.5 filter in a three-electrode cell (0.5 M Na2SO4). Ag/AgCl electrode and Pt wire were used as reference and counter electrodes, respectively.
3 Results and Discussion
A typical synthesis route of 1T-WS2@TiO2@Ti3C2 composites is schematically depicted in Scheme 1. Ti3C2 MXenes are firstly prepared by etching Al layers of Ti3AlC2 MAX phase in HF solution [27]. Then, the layered Ti3C2 MXene provides Ti sources with the help of HCl and NaBF4 for growing TiO2 NSs across the layered Ti3C2 MXene. Finally, the obtained TiO2@Ti3C2 composites are added into WCl6/TAA solutions at 200 °C for 24 h to introduce 1T-WS2 co-catalysts. In this process, due to the intercalation of NH4+ of TAA, the space distance of WS2 increases and 1T-WS2 is generated [19]. The 1T-WS2 is evenly assembled on TiO2@Ti3C2 composites’ surface to construct the ternary 1T-WS2@TiO2@Ti3C2 composites.
After HF etching, the most intense XRD (104) peak of Ti3AlC2 was disappeared, and the (002) peak of Ti3AlC2 at 9.52o was moved to lower 2-theta value (8.78o), which indicates the successful formation of Ti3C2 (Fig. S1a) [17]. The development of TiO2 nanosheets across Ti3C2 MXenes by the hydrothermal oxidation of Ti3C2 is evidenced by the emergence of diffraction peaks of anatase TiO2 (JCPDS No. 21-1272) as shown in Fig. 1a. The XRD peaks appearing at 6.66°, 13.40°, and 20.10° are indexed to (002), (004), and (006) planes of 1T-WS2 [23]. The co-existence of Ti3C2, TiO2, and 1T-WS2 indicates the successful preparation of 1T-WS2@TiO2@Ti3C2 composites. For 1T-WS2@TiO2@Ti3C2 composites with other WS2 ratios (Fig. S1b), all the XRD peaks are well corresponding to Ti3C2, TiO2, or 1T-WS2.
The full-scale XPS spectrum of 1T-WS2@TiO2@Ti3C2 composites (Fig. 1b) displays that Ti, C, O, S, and W are dominant elements, while F element is ascribed to F− ions physically adsorbed on composites from the HF solution. The Ti 2p spectrum is divided into four peaks (Fig. 1c). The two peaks at 464.7 (Ti–O 2p1/2) and 459.0 eV (Ti–O 2p3/2) are ascribed to lattice Ti–O bonds of TiO2 [28]. The other two peaks at 461.2 (Ti-C 2p1/2) and 455.3 eV (Ti-C 2p3/2) are indexed to lattice Ti–C bonds of Ti3C2 [29]. The high-resolution Ti 2p XPS spectrum indicates the content of Ti3C2 is about 79% in TiO2@Ti3C2 composites. The W 4f XPS spectrum of 1T-WS2@TiO2@Ti3C2 composites (Fig. 1d) can confirm the presence and relative content of 1T-WS2. In the W 4f region, the two peaks of 2H phase corresponding to W 4f7/2 and W 4f5/2 at 33.0 and 36.0 eV, respectively. Nevertheless, two extra peaks shift to lower binding energies at 32.4 and 34.6 eV, suggesting the existence of 1T-WS2 [19, 30]. The 1T phase content is calculated about 73%, which shows that the 1T-WS2@TiO2@Ti3C2 composites are composed of lots of metallic 1T phase.
Ti3C2 MXenes are obtained by etching of the aluminum layer of the bulk Ti3AlC2 (Fig. S2a) by using HF. As shown in Fig. 2a, Ti3C2 MXenes present typical accordion-like multilayer structure. After hydrothermal oxidation of Ti3C2 MXenes, the layered Ti3C2 MXenes provide Ti sources for growing TiO2 NSs inserting across the layered Ti3C2 MXene to form TiO2@Ti3C2 composites, and further combine with WS2 through hydrothermal reaction to get 1T-WS2@TiO2@Ti3C2 composites. Figure 2b shows that 1T-WS2 presents nanoflake structures and agglomerate into nanoflowers. After combining with TiO2@Ti3C2 composites, the 1T-WS2 nanoparticles are evenly distributed on TiO2@Ti3C2 composites’ surface (Fig. 2c, d). Furthermore, 1T-WS2@TiO2@Ti3C2 composites with other WS2 ratios (10, 20, and 25 wt%) are prepared, and corresponding SEM images are displayed in Fig. S2.
The phase composition and microscopic structure of 1T-WS2@TiO2@Ti3C2 composites are characterized by TEM (Fig. 3a, b). The lattice with d spaces of 0.35 nm is attributed to (101) plane of anatase TiO2 (Fig. 3b), which are same as the description of the literatures [31, 32]. The lattice spacings of 0.98 and 0.92 nm are indexed to (002) plane of Ti3C2 MXene and (002) plane of 1T-WS2 [19, 33,34,35] (Fig. 3b). The EDX element mappings of composites (Fig. 3c) indicate that the Ti, C, O, W, and S elements are accordantly distributed. The as-fabricated photocatalyst with superior metallic quality of Ti3C2 MXene and 1T-WS2 present more effective carrier transfer and separation compared with TiO2 NSs, and therefore, the photocatalytic performance is enhanced. The 1T-WS2 is further confirmed by Raman spectroscopy (Fig. S3). Remarkably, in contrast to 2H phase WS2, there are no scattering peaks between 350 and 450 cm−1 attributed to \(E_{2g}^{1}\) (in-plane) and A1g (out-of-plane) in 1T-WS2 (Fig. S3). There are also two strong peaks at low frequency range for 1T-WS2. One strong Raman band at 128 cm−1 (J1) is attributed to W–W stretching vibrations in 1T-WS2@TiO2@Ti3C2 composite [21]. Besides, another additional peak at 171 cm−1 (J2) is observed, which is associated with the phonon modes in the WS2, suggesting the existence of a considerable amount of 1T phase ingredient embedded [22]. This result further implies that the as-prepared WS2 in 1T-WS2@TiO2@Ti3C2 composites is mostly 1T phase [19, 23].
To further examine the textural properties of 1T-WS2@TiO2@Ti3C2 composites, the isotherms and the pore size distributions are studied by N2 adsorption–desorption measurement (Figs. 4 and S4). All of samples present type IV isotherms with H3 hysteresis loops, suggesting the presence of mesopores [36]. And the pore size distribution curves of 1T-WS2@TiO2@Ti3C2 composites with different WS2 loading amounts (Fig. 4a–d inset) display that the size of major mesopores ranges from 2 to 25 nm. Compared with other samples, when the loading amount of WS2 is 15%, the pore size distribution is relatively concentrated at 2 ~ 5 nm. The presence of such a small pore size is conducive to migration of reactant and product molecules to facilitate photocatalytic reactions. Moreover, larger nitrogen adsorption capacity indicates that more reactive sites may be provided during the reaction process, which is favorable in the enhancement of catalytic activity. The BET surface area of as-prepared 1T-WS2@TiO2@Ti3C2-15%, as shown in Table S1, reveals a higher surface area (23.334 m2 g−1) than those of Ti3C2 MXene, pure 1T-WS2, and 1T-WS2@TiO2@Ti3C2 composites with other WS2 loading amounts. In addition, as shown in Figs. 4 and S4, the BET surface area of the samples increase nonlinearly with increasing WS2 loading. As the WS2 loading amount increases from 10 to 15 wt%, the BET surface area of composites increases. However, further increasing the loading amount of WS2 (from 15 to 25 wt% WS2) leads to a gradual decrease of BET surface area, which may be caused by the aggregation of WS2 on the surface of the composites. The higher surface areas are beneficial for photocatalysis since it could provide more adsorption and active sites, thus the photocatalytic activity is improved [37].
To investigate the optical absorptivity, the UV–Vis DRS spectra of samples are measured. As shown in Fig. 5, TiO2 NSs (curve black) have a noticeable UV light absorption, due to the nature of anatase TiO2 [38]. Ti3C2 MXene (curve blue) shows UV and visible absorption as a result of the black color nature [39]. Compared with TiO2 NSs, 1T-WS2@TiO2@Ti3C2 composites (15 wt% WS2) display a significant absorption edge red shift and enhanced visible absorption, which is attributed to the optical absorption of Ti3C2 MXene and 1T-WS2. The increase of light absorption range of photocatalysts will be more helpful to promote the progress of photocatalytic reaction. Besides, the 1T-WS2@TiO2@Ti3C2 composites show stronger light absorption with the increase of WS2 contents from 10 to 25 wt% (Fig. S5).
The photocatalytic performance of 1T-WS2@TiO2@Ti3C2 composites was evaluated using H2 evolution under simulated sunlight irradiation in an aqueous acetone solution at room temperature (Fig. 6). Control experiments (Fig. S6) show that no noticeable H2 evolution is discovered without either photocatalyst or illumination. TiO2 NSs (Fig. 6) present limited photocatalytic H2 activity (67.8 μmol g−1 h−1), arising from fast carrier recombination [40]. In view of the excellent electronic conductivity of Ti3C2 and 1T-WS2, it is combined as a co-catalyst with TiO2 NSs in order to achieve better photogenerated carrier separation and improve photocatalytic performance [41, 42]. As expected, after assembling of Ti3C2 and 1T-WS2, the bets photocatalytic activity is detected (3409.8 μmol g−1 h−1 for 1T-WS2@TiO2@Ti3C2 composites (15 wt% WS2)), which is nearly 50 times higher than that of TiO2 NSs. Moreover, the 1T-WS2@TiO2@Ti3C2 composites (10 wt% WS2) present a lower photocatalytic activity than 1T-WS2@TiO2@Ti3C2 composites (15 wt% WS2), owing to the relatively weaker solar light input. Furthermore, with the increase of WS2 contents from 15 to 25 wt%, a reduction in the photocatalytic performance of 1T-WS2@TiO2@Ti3C2 composites is discovered. Because excess black 1T-WS2 nanoparticles induced “shielding effect” block light to the surface of TiO2 [43].
Besides, we evaluate the apparent quantum efficiency (AQE) of photocatalysts under the same light source. Table S2 displays the comparison of AQE values of TiO2 NSs and 1T-WS2@TiO2@Ti3C2 composites with different WS2 ratios (10, 15, 20, and 25 wt%): 0.049% (TiO2 NSs) < 1.173% (1T-WS2@TiO2@Ti3C2-25 wt%) < 1.513% (1T-WS2@TiO2@Ti3C2-10 wt%) < 1.956% (1T-WS2@TiO2@Ti3C2-20 wt%) < 2.464% (1T-WS2@TiO2@Ti3C2-15 wt%), which is in accordance with photocatalytic H2 evolution performance. Moreover, we conduct stability of 1T-WS2@TiO2@Ti3C2 composites (15 wt% WS2) for 24 h (Fig. S7). No noticeable H2 production decrease is detected after 3 cycles (24 h). SEM images (Fig. S8) and XRD pattern (Fig. S9) of 1T-WS2@TiO2@Ti3C2 composites after 3 cycles display no evident difference compared with fresh samples. The results further demonstrate that 1T-WS2@TiO2@Ti3C2 composites can act as a favorable photocatalyst for H2 production. We also delaminated the multilayered Ti3C2 MXenes to get monolayered Ti3C2 nanosheets (Fig. S10). The XRD pattern of TiO2@Ti3C2 (monolayer) does not detect the diffraction peak of Ti3C2 (Fig. S10a). Since Ti3C2 monolayer is in full contact with the reaction solution, all Ti3C2 may be converted into TiO2 under the same experimental conditions. As shown in Fig. S10b, 1T-WS2@Ti3C2@Ti3C2 (monolayer) presents worse photocatalytic H2 production activity than that of 1T-WS2@TiO2@Ti3C2 (multilayer), which demonstrates that the lack of Ti3C2 by oxidation in 1T-WS2@Ti3C2@Ti3C2 (monolayer) greatly affects the photocatalytic H2 production. Furthermore, changing the ratio between Ti3C2 and TiO2 also affecting the photocatalytic performance of 1T-WS2@TiO2@Ti3C2 composites (Fig. S11). Compared with in situ loading of TiO2 nanosheets (Fig. S12), foreign titanium sources do not improve the photocatalytic performance of 1T-WS2@TiO2@Ti3C2 composites, which may be caused by the non-close contact between TiO2 and Ti3C2 caused by the foreign titanium sources.
The introduction of Ti3C2 MXene and 1T-WS2 in 1T-WS2@TiO2@Ti3C2 composites would be believed to influence photoinduced carrier separation, which could be characterized by steady and time-resolved PL spectroscopy (Fig. 7). As illustrated in Fig. 7a, TiO2 NSs possess a high PL peak, resulting in the quick photoinduced carrier recombination. When Ti3C2 MXene and 1T-WS2 are incorporated, the PL peak is significantly reduced (Fig. 7a). Evidently, the photoinduced carrier recombination of TiO2 is hindered by migrating electrons to Ti3C2 and 1T-WS2 as electron acceptors [44]. An increased lifetime of charge carriers is also detected by loading Ti3C2 MXene and 1T-WS2 (Fig. 7b). The intensity-average lifetimes (τ) of TiO2 NSs are 0.1138 ns, much shorter than that of 1T-WS2@TiO2@Ti3C2 composites (1.2750 ns). The increased carrier lifetime of 1T-WS2@TiO2@Ti3C2 composites is beneficial for enhanced carrier separation efficiency.
The photocurrent responses of photocatalysts were prompt by some on–off cycles under light illumination (Fig. 8a). All of samples present reversible photocurrent responses on each irradiation. The photocurrent intensity of 1T-WS2@TiO2@Ti3C2 composites is much higher than that of pure TiO2 NSs, which is due to Ti3C2 and 1T-WS2 as co-catalysts more effectively receiving photoexcited electrons of TiO2. The 1T-WS2@TiO2@Ti3C2 composites (Fig. 8b) exhibit a smaller arc radius compared with TiO2 NSs under light irradiation, suggesting that the 1T-WS2@TiO2@Ti3C2 composite presents smaller charge transfer resistance, finally causing higher photoexcited carrier transfer and separation efficiency [45].
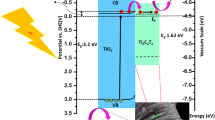
As shown in Scheme 2, under light irradiation, TiO2 NSs can be excited to produce electrons and holes. The majority of photoexcited electrons in conduction band (CB) of TiO2 could instantly migrate to metallic Ti3C2 MXene and 1T-WS2 through the interface. As the photoelectron receivers, Ti3C2 MXene and 1T-WS2 serves as active sites for H2 production [46, 47]. Meanwhile, the holes in the valence band (VB) of TiO2 are consumed by the sacrificial reagents. Consequently, the photoexcited carriers are efficiently transferred and separated with the assistance of double co-catalysts Ti3C2 and 1T-WS2.
4 Conclusions
In conclusion, an effective 1T-WS2@TiO2@Ti3C2 composite photocatalyst is successfully prepared. The development of TiO2 NSs on Ti3C2 MXenes and 1T-WS2 nanoparticles uniformly distributing on TiO2@Ti3C2 composite is the design concept. The obtained 1T-WS2@TiO2@Ti3C2 composite with 15 wt% WS2 loading displays excellent photocatalytic H2 production performance (3409.8 μmol g−1 h−1), nearly 50 times higher than that of pure TiO2 NSs. The excellent H2 evolution performance of 1T-WS2@TiO2@Ti3C2 composites is ascribed to the following reasons: (1) The introduction of 1T-WS2 nanoparticles induces enhanced BET surface area and more active sites; (2) Both Ti3C2 MXene and 1T-WS2 possess extraordinary conductivity, which greatly enhance the electron transfer ability and thus achieve highly efficient spatial charge separation.
References
S.W. Boettcher, E.L. Warren, M.C. Putnam, E.A. Santori, D.T. Evans et al., Photoelectrochemical hydrogen evolution using Si microwire arrays. J. Am. Chem. Soc. 133, 1216–1219 (2011). https://doi.org/10.1021/ja108801m
J. Choi, S.Y. Ryu, W. Balcerski, T.K. Lee, M.R. Hoffmann, Photocatalytic production of hydrogen on Ni/NiO/KNbO3/CdS nanocomposites using visible light. J. Mater. Chem. 18, 2371–2378 (2008). https://doi.org/10.1039/B718535A
M.R. Gholipour, C.T. Dinh, F. Béland, T.O. Do, Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 7, 8187–8208 (2015). https://doi.org/10.1039/C4NR07224C
Z. Li, B. Tian, W. Zhen, Y. Wu, G. Lu, Inhibition of hydrogen and oxygen recombination using oxygen transfer reagent hemin chloride in Pt/TiO2 dispersion for photocatalytic hydrogen generation. Appl. Catal. B-Environ. 203, 408–415 (2017). https://doi.org/10.1016/j.apcatb.2016.10.049
Z. Liang, X. Bai, P. Hao, Y. Guo, Y. Xue, J. Tian, H. Cui, Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B 243, 711–720 (2019). https://doi.org/10.1016/j.apcatb.2018.11.017
X. Zhang, Y. Wang, B. Liu, Y. Sang, L. Hong, Heterostructures construction on TiO2 nanobelts: a powerful tool for building high-performance photocatalysts. Appl. Catal. B 202, 620–641 (2017). https://doi.org/10.1016/j.apcatb.2016.09.068
Y. Cao, Y. Saygili, A. Ummadisingu, J.L. Teuscher, J. Luo, N. Pellet, F. Giordano, S.M. Zakeeruddin, J.E. Moser, M. Freitag, 11% efficiency solid-state dye-sensitized solar cells with copper (II/I) hole transport materials. Nat. Commun. 8, 15390 (2017). https://doi.org/10.1038/ncomms15390
N. Michael, Alkaline earth metal oxide nanocluster modification of rutile TiO2 (110) promotes water activation and CO2 chemisorption. J. Mater. Chem. A 6, 9451–9466 (2018). https://doi.org/10.1039/C8TA01789A
J. Jiao, Y. Wei, Y. Zhao, Z. Zhao, A. Duan et al., AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: high efficient separation of photogenerated charge carriers. Appl. Catal. B 209, 228–239 (2017). https://doi.org/10.1016/j.apcatb.2017.02.076
S. Ma, Y. Deng, J. Xie, K. He, W. Liu, X. Chen, X. Li, Noble-metal-free Ni3C cocatalysts decorated CdS nanosheets for high-efficiency visible-light-driven photocatalytic H2 evolution. Appl. Catal. B 227, 218–228 (2018). https://doi.org/10.1016/j.apcatb.2018.01.031
Y.J. Yuan, D. Chen, J. Zhong, L.X. Yang, J. Wang et al., Interface engineering of a noble-metal-free 2D-2D MoS2/Cu-ZnIn2S4 photocatalyst for enhanced photocatalytic H2 production. J. Mater. Chem. A 5, 15771–15779 (2017). https://doi.org/10.1039/C7TA04410K
Y.J. Yuan, H.W. Lu, Z.T. Yu, Z.G. Zou, Noble-metal-free molybdenum disulfide cocatalyst for photocatalytic hydrogen production. Chemsuschem 8, 4113–4127 (2016). https://doi.org/10.1002/cssc.201501203
Y. Sun, X. Meng, Y. Dall’Agnese, C. Dall’Agnese, S. Duan, Y. Gao, G. Chen, X.F. Wang, 2D MXenes as co-catalysts in photocatalysis: synthetic methods. Nano-Micro Lett. 11, 79 (2019). https://doi.org/10.1007/s40820-019-0309-6
M. Naguib, V.N. Mochalin, M.W. Barsoum, Y. Gogotsi, 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv. Mater. 26, 992–1005 (2014). https://doi.org/10.1002/adma.201304138
Z. Li, F. Wu, J. Yu, Q. Deng, F. Zhang, W. Guan, Titanium carbide (Ti3C2Tx) MXene: a novel precursor to amphiphilic carbide-derived graphene quantum dots for fluorescent ink, light-emitting composite and bioimaging. Carbon 118, 50–57 (2017). https://doi.org/10.1016/j.carbon.2017.03.023
X. Xie, M.Q. Zhao, B. Anasori, K. Maleski, C.E. Ren, J. Li, B.W. Byles, E. Pomerantseva, G. Wang, Y. Gogotsi, Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 26, 513–523 (2016). https://doi.org/10.1016/j.nanoen.2016.06.005
Y. Li, X. Deng, J. Tian, Z. Liang, H. Cui, Ti3C2 MXene-derived Ti3C2/TiO2 nanoflowers for noble-metal-free photocatalytic overall water splitting. Appl. Mater. Today 13, 217–227 (2018). https://doi.org/10.1016/j.apmt.2018.09.004
J. Pang, R.G. Mendes, A. Bachmatiuk, L. Zhao, H.Q. Ta et al., Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 48, 72–133 (2019). https://doi.org/10.1039/C8CS00324F
J. Yi, X. She, Y. Song, M. Mao, K. Xia et al., Solvothermal synthesis of metallic 1T-WS2: a supporting co-catalyst on carbon nitride nanosheets toward photocatalytic hydrogen evolution. Chem. Eng. J. 335, 282–289 (2018). https://doi.org/10.1016/j.cej.2017.10.125
J.M. Woods, Y. Jung, Y. Xie, W. Liu, Y. Liu, H. Wang, J.J. Cha, One-step synthesis of MoS2/WS2 layered heterostructures and catalytic activity of defective transition metal dichalcogenide films. ACS Nano 10, 2004–2009 (2016). https://doi.org/10.1021/acsnano.5b06126
X. Tong, Y. Qi, J. Chen, N. Wang, Q. Xu, Supercritical CO2-assisted reverse-micelle-induced solution-phase fabrication of two-dimensional metallic 1T-MoS2 and 1T-WS2. ChemNanoMat 3, 466–471 (2017). https://doi.org/10.1002/cnma.201700011
M. Piao, J. Chu, X. Wang, Y. Chi, H. Zhang, C. Li, H. Shi, M.K. Joo, Hydrothermal synthesis of stable metallic 1T phase WS2 nanosheets for thermoelectric application. Nanotechnology 29, 025705 (2017). https://doi.org/10.1088/1361-6528/aa9bfe
Q. Liu, X. Li, Z. Xiao, Y. Zhou, H. Chen et al., Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions: the correlation between structure and electrical/optical properties. Adv. Mater. 27, 4837–4844 (2015). https://doi.org/10.1002/adma.201502134
U. Maitra, U. Gupta, M. De, R. Datta, A. Govindaraj, C.N. Rao, Highly effective visible-light-induced H2 generation by single-layer 1T-MoS2 and a nanocomposite of few-layer 2H-MoS2 with heavily nitrogenated graphene. Angew. Chem. Int. Ed. 52, 13057–13061 (2013). https://doi.org/10.1002/anie.201306918
H. Yu, P. Xiao, P. Wang, J. Yu, Amorphous molybdenum sulfide as highly efficient electron-cocatalyst for enhanced photocatalytic H2 evolution. Appl. Catal. B 193, 217–225 (2016). https://doi.org/10.1016/j.apcatb.2016.04.028
X.B. Li, Y.J. Gao, H.L. Wu, Y. Wang, Q. Guo et al., Assembling metallic 1T-MoS2 nanosheets with inorganic-ligand stabilized quantum dots for exceptional solar hydrogen evolution. Chem. Commun. 53, 5606–5609 (2017). https://doi.org/10.1039/C7CC02366A
Y. Li, Z. Yin, G. Ji, Z. Liang, Y. Xue, Y. Guo, J. Tian, X. Wang, H. Cui, 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B 246, 12–20 (2019). https://doi.org/10.1016/j.apcatb.2019.01.051
M.A. Lukowski, A.S. Daniel, F. Meng, A. Forticaux, L. Li, S. Jin, Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013). https://doi.org/10.1021/ja404523s
M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, H. Min, L. Hultman, Y. Gogotsi, M.W. Barsoum, Two-dimensional nanocrystals: two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 37, 4207 (2011). https://doi.org/10.1002/adma.201190147
C.C.M. Martinez, A. Ambrosi, A.Y.S. Eng, Z. Sofer, M. Pumera, Metallic 1T-WS2 for selective impedimetric vapor sensing. Adv. Funct. Mater. 25, 5611–5616 (2015). https://doi.org/10.1002/adfm.201502223
J. Tian, X. Hu, N. Wei, Y. Zhou, X. Xu, H. Cui, H. Liu, RuO2/TiO2 nanobelt heterostructures with enhanced photocatalytic activity and gas-phase selective oxidation of benzyl alcohol. Sol. Energ. Mat. Sol. C 151, 7–13 (2016). https://doi.org/10.1016/j.solmat.2016.02.017
X. Hu, S. Lu, J. Tian, N. Wei, X. Song, X. Wang, H. Cui, The selective deposition of MoS2 nanosheets onto (101) facets of TiO2 nanosheets with exposed (001) facets and their enhanced photocatalytic H2 production. Appl. Catal. B 241, 329–337 (2019). https://doi.org/10.1016/j.apcatb.2018.09.051
P. Lian, Y. Dong, Z. Wu, S. Zheng, X. Wang, S. Wang, C. Sun, J. Qin, X. Shi, X. Bao, Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy 40, 1–8 (2017). https://doi.org/10.1016/j.nanoen.2017.08.002
T.A.J. Loh, D.H.C. Chua, Origin of hybrid 1T- and 2H-WS2 ultrathin layers by pulsed laser deposition. J. Phys. Chem. C 49, 27496–27504 (2015). https://doi.org/10.1021/acs.jpcc.5b09277
B. Mahler, V. Hoepfner, K. Liao, G.A. Ozin, Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: aplications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 136, 14121–14127 (2014). https://doi.org/10.1021/ja506261t
F. Yang, Z. Ning, H. Liu, Fractal characteristics of shales from a shale gas reservoir in the Sichuan Basin. China Fuel 115, 378–384 (2014). https://doi.org/10.1016/j.fuel.2013.07.040
Y. Cui, G. Zhang, Z. Lin, X. Wang, Condensed and low-defected graphitic carbon nitride with enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B 181, 413–419 (2016). https://doi.org/10.1016/j.apcatb.2015.08.018
H. Yang, J. Tian, Y. Bo, Y. Zhou, X. Wang, H. Cui, Visible photocatalytic and photoelectrochemical activities of TiO2 nanobelts modified by In2O3 nanoparticles. J. Colloid Interf. Sci. 487, 258–265 (2017). https://doi.org/10.1016/j.jcis.2016.10.051
J. Ran, G. Gao, F. Li, T. Ma, A. Du, S. Qiao, Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 8, 13907 (2017). https://doi.org/10.1038/ncomms13907
L. Yao, N. Zhang, Y. Wang, Y. Ni, Facile formation of 2D Co2P@Co3O4 microsheets through in situ toptactic conversion and surface corrosion: bifunctional electrocatalysts towards overall water splitting. J. Power Sources 374, 142–148 (2018). https://doi.org/10.1016/j.jpowsour.2017.11.028
A.N. Enyashin, A.L. Ivanovskii, Structural and electronic properties and stability of MXenes Ti2C and Ti3C2 functionalized by methoxy groups. J. Phys. Chem. C 117, 13637–13643 (2013). https://doi.org/10.1021/jp401820b
T.A.J. Loh, M. Tanemura, D.H.C. Chua, Ultrathin MoS2 and WS2 layers on silver nano-tips as electron emitters. Appl. Phys. Lett. 109, 133102 (2016). https://doi.org/10.1063/1.4963260
H.E. Kim, J. Lee, H. Lee, C. Lee, Synergistic effects of TiO2 photocatalysis in combination with fenton-like reactions on oxidation of organic compounds at circumneutral pH. Appl. Catal. B 116, 219–224 (2012). https://doi.org/10.1016/j.apcatb.2011.12.027
M. Schvartzman, V. Sidorov, D. Ritter, Y. Paz, Surface passivation of (100) InP by organic thiols and polyimide as characterized by steady-state photoluminescence. Semicond. Sci. Technol. 16, 68–71 (2001). https://doi.org/10.1088/0268-1242/16/10/103
Y. Liu, W. Wang, Y. Wang, X. Peng, Homogeneously assembling like-charged WS2 and GO nanosheets lamellar composite films by filtration for highly efficient lithium ion batteries. Nano Energy 7, 25–32 (2014). https://doi.org/10.1016/j.nanoen.2014.04.018
A. Manikandan, P.R. Ilango, C.W. Chen, Y.C. Wang, Y.C. Shih et al., A superior dye adsorbent towards the hydrogen evolution reaction combining active sites and phase-engineering of (1T/2H) MoS2/α-MoO3 hybrid heterostructured nanoflowers. J. Mater. Chem. A 6, 15320 (2018). https://doi.org/10.1039/C8TA02496K
X. An, W. Wang, J. Wang, H. Duan, J. Shi, X. Yu, The synergetic effects of Ti3C2 MXene and Pt as co-catalysts for highly efficient photocatalytic hydrogen evolution over g-C3N4. Phys. Chem. Chem. Phys. 20, 11405–11411 (2018). https://doi.org/10.1039/C8CP01123K
Acknowledgements
The authors are thankful for fundings from the National Natural Science Foundation of China (Nos. 51872173 and 51772167), Taishan Scholarship of Young Scholars (No. tsqn201812068), Natural Science Foundation of Shandong Province (No. ZR2017JL020), Taishan Scholarship of Climbing Plan (No. tspd20161006), and Key Research and Development Program of Shandong Province (No. 2018GGX102028).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Ding, L., Yin, S. et al. Photocatalytic H2 Evolution on TiO2 Assembled with Ti3C2 MXene and Metallic 1T-WS2 as Co-catalysts. Nano-Micro Lett. 12, 6 (2020). https://doi.org/10.1007/s40820-019-0339-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-019-0339-0