Abstract
Introduction
Lupus nephritis (LN) treatment aims to control and prevent flares and irreversible kidney damage. Around 30% of patients are unresponsive to treatment; however, real-world LN treatment patterns have not been reported. Objectives of this retrospective cohort study (GSK 209758) were to quantify the time to switching/re-initiating induction therapy in patients with LN initiating immunosuppressant therapy and conversion from induction to maintenance immunosuppressant therapy, and to assess corticosteroid use.
Methods
Patients with LN initiating induction or maintenance immunosuppressant therapy were identified using claims data. Patients were followed up from the index date (immunosuppressant initiation date) until treatment discontinuation, death, disenrollment, administrative censoring, or the end of follow-up period. The cumulative incidence of switching/re-initiating induction therapy and conversion to maintenance therapy was estimated using outpatient pharmacy claims and procedure codes. Corticosteroid use was estimated using pharmacy claims; a mean daily dose of ≥ 7.5 mg/day was considered high.
Results
In total, 5000 patients with LN contributed 5516 treatment episodes (induction cohort, N = 372; maintenance cohort, N = 5144). In the induction cohort, the cumulative incidence (95% confidence interval) of switching between induction therapies was 24.6% (20.1–30.0) at 12 months, while 59.6% (52.4–66.1) of patients converted to maintenance therapy at 12 months. In the maintenance cohort, 21.2% (19.9–22.5) re-initiated induction therapy at 12 months. Oral corticosteroid use decreased during the follow-up in both cohorts, but 21.5% of patients remained on a high dose at 12 months in the induction cohort, while 15.8% in the maintenance cohort were taking a high dose at 24 months.
Conclusions
Around a quarter of patients with LN initiating immunosuppressant therapy switched within 12 months, while a fifth re-initiated induction therapy within 12 months. Use of high corticosteroid doses were observed during 24 months of follow-up. These data suggest that many patients do not respond to existing standard LN therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Lupus nephritis (LN) occurs in approximately 40% of patients with systemic lupus erythematosus; of these, around 30% are unresponsive to current treatments. |
Reports of the real-world treatment patterns of LN are limited, but are critical in understanding the current unmet need for effective treatments for patients with LN. |
This retrospective cohort study aimed to quantify the time to switching/re-initiating induction therapy and conversion to maintenance therapy, and to assess corticosteroid use among patients with LN. |
What was learned from the study? |
Around a quarter of patients with LN initiating immunosuppressant therapy switched treatment within 12 months, while a fifth re-initiated induction therapy; approximately a fifth received high doses (≥ 7.5 mg/day) of corticosteroids at 12 months of follow-up. |
These findings suggest that many patients do not respond to the current standard LN therapies and that new treatments that improve response and prevent renal flares are needed. |
Introduction
Lupus nephritis (LN) is a severe manifestation of systemic lupus erythematosus (SLE) and occurs in approximately 40% of patients with SLE [1]. Currently, there are no curative therapies available for patients with LN; treatment largely focusses on managing the disease, for example, by controlling ongoing glomerular inflammation and reducing tissue injury, in order to prevent renal flares/relapses and associated irreversible kidney damage requiring dialysis and transplant [2].
The 2012 American College of Rheumatology (ACR) guidelines for the treatment of LN recommend a period of induction therapy (6 months) with immunosuppressive agents (cyclophosphamide [CYC] or mycophenolate mofetil [MMF]) in combination with corticosteroids; this is followed by maintenance treatment with either MMF alone, or azathioprine (AZA) with or without use of corticosteroids for patients that respond to induction immunosuppressant therapy [3]. For patients not responding to initial induction immunosuppressant therapy after 6 months, a treatment switch from MMF to CYC and vice versa in combination with corticosteroids is recommended [3]. Rituximab can also be considered for patients who do not improve (or worsen) after 6 months of induction therapy with either MMF or CYC, or both [3]. While consensus on the use of calcineurin inhibitors was not reached during development of the ACR guidelines, calcineurin inhibitors in combination with MMF may also be considered in patients who do not respond to induction therapy [3]. Treatment guidelines issued by the European Alliance of Associations for Rheumatology and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommend similar treatment regimens [2]; these guidelines also indicate that belimumab can be considered as an add-on treatment in patients with refractory disease [2]. Since the development of these guidelines, belimumab has been approved by the United States (US) Food and Drug Administration (FDA) for the treatment of active LN among patients receiving standard therapy [4].
Commonly used treatments including MMF and CYC are not entirely effective. One clinical trial showed that 23% and 6% of patients receiving MMF and CYC, respectively, achieved complete remission at 24 weeks [5], while another showed that 9% and 8% achieved complete remission after 24 weeks, respectively [6]. A prospective, 5-year study reported that 51% of patients remained in complete remission and 33% of patients failed treatment with MMF at the end of follow-up [7]. Moreover, many patients that achieve remission will experience a relapse during maintenance therapy [8]. In longitudinal registries, approximately 10–20% of patients with LN have progressed to end-stage kidney disease within 10 years [1, 9, 10].
Additionally, these therapies have documented toxicities. Use of CYC is associated with bladder toxicity while MMF can cause side effects associated with the gastrointestinal, hematological, and genitourinary systems, and is a risk factor for herpes zoster infection [11,12,13,14]. Moreover, the use of immunosuppressants (including CYC and MMF) is associated with increased risk of serious infection [15, 16]. Sustained use of corticosteroids is also associated with adverse outcomes including irreversible organ damage [17,18,19]. These observations highlight the wide burden of unmet need within the current treatment paradigm. However, descriptions of real-world treatment patterns of patients with LN are limited, preventing a full understanding of the true unmet need in this population.
A greater understanding of real-world treatment paradigms will facilitate optimization of future treatment regimens and improve outcomes. Key unanswered questions include: how frequently (and when) do patients switch between, or re-initiate, induction immunosuppressant therapies? What proportion of patients successfully transition from induction to maintenance therapy and when? How many patients are receiving high doses of corticosteroids while using immunosuppressants in routine practice?
Analyses of data from administrative claims databases provide a means of exploring these questions in a real-world setting. Data are collected for non-research purposes, primarily billing, and are therefore limited in detail, as they only capture information sufficient for managing insurance claims. These databases provide access to a wealth of information from a large and demographically diverse cohort, but rarely include the detailed clinical information that is found in electronic medical records or clinical registries [20].
The purpose of this study was to characterize the treatment patterns in the routine care setting for patients with LN. The primary objective was to quantify the time from initiating induction or maintenance therapy to switching or re-initiating induction therapy, respectively. Secondary objectives described here were to quantify the time to conversion to maintenance therapy among patients who had initiated induction therapy; and to characterize the proportion of patients receiving high mean daily doses of corticosteroids while using immunosuppressants.
Methods
Study Design and Data Source
This was a retrospective cohort study (GSK Study 209758) using insurance claims data collected in 2012–2019 from the IBM MarketScan Commercial Claims and Encounters database. This database is de-identified and consists of medical (inpatient and outpatient) and prescription drug data from employers and health plans for over 201 million individuals in the US from its inception until 2020.
The study design is shown as a scheme in Supplementary Fig. S1. Adult patients with LN who initiated an immunosuppressant of interest (CYC, MMF, or AZA) were categorized into two cohorts. The ‘induction immunosuppressant’ cohort included patients initiating CYC with pulse corticosteroids or MMF with pulse corticosteroids. The ‘maintenance immunosuppressant’ cohort included patients initiating MMF without pulse corticosteroids or AZA with or without corticosteroids (referred to herein as MMF and AZA, respectively).
The immunosuppressant initiation date (i.e., the index date) was defined as the first date that the patient was administered or filled a prescription for an immunosuppressant of interest, with no evidence of previously receiving that study medication in the previous 6 months and meeting all other inclusion/exclusion criteria. Receipt of pulse corticosteroids was assessed in the 14 days prior to and following the prescription date for each immunosuppressant. Patients were followed up from the index date until treatment discontinuation (defined below), death, disenrollment, administrative censoring (end of available data on 31 December, 2019) or the end of the follow-up period (12 months for induction immunosuppressant cohort and 48 months for maintenance immunosuppressant cohort); additional outcome-specific criteria for follow-up periods were also applied (see “Variables and Outcomes”).
Discontinuation was defined based on how the initiated treatment was identified. For study treatments identified with prescription claims, patients were classified as having discontinued treatment if they did not receive a subsequent prescription within the days’ supply provided in the pharmacy claim, plus a 90-day grace period. Alternatively, if a patient initiated CYC in an inpatient or outpatient setting (with no available data on days’ supply provided in the claim), they were assumed to take the treatment for 30 days based on typical dosing patterns for this medication, with a similar 90-day grace period allowed between infusions before patients were classified as having discontinued treatment [3].
Patients could contribute up to four treatment episodes if they met the eligibility criteria described below for each immunosuppressant regimen of interest.
Study Population
Patients were eligible for inclusion in the study if they were prescribed an immunosuppressant of interest; had documented LN in the 12 months preceding index (defined by the presence of ≥ 1 International Classification of Diseases 10th revision [ICD-10] LN code, or ≥ 1 ICD 9th revision [ICD-9]/ICD-10 SLE code and ≥ 2 nephritis codes recorded ≥ 30 days apart); and had 12 months of medical and drug coverage preceding the index date.
Patients were excluded if they had received the same immunosuppressant that they were prescribed on the index date during the 6 months preceding index (washout period); were simultaneously prescribed multiple study medications on the index date; or were < 18 years of age on the index date.
Ethics Declaration
No direct subject contact or primary collection of individual human subject data occurred. Study results omit subject identification; therefore, informed consent, ethics committee, or Institutional Review Board approval was not required. The study data are available from IBM MarketScan Commercial Claims and Encounters database, but restrictions apply to the availability of these data, which were used under license for the current study; GSK had permission to access the database.
Variables and Outcomes
Demographic and Clinical Characteristics
Patient demographics and clinical characteristics were assessed on the index date or during the 12 months preceding the index date. SLE-related extra-renal conditions (e.g., arthritis/arthralgia, myalgia/myositis, pleurisy/pleural effusion and rash) were identified based on the presence of specific ICD-9 and ICD-10 codes. Details regarding the inclusion of SLE-related conditions are shown in the Supplementary Materials. Prior use of SLE-related medications of interest (including immunosuppressants and antimalarials) was determined based on pharmacy claims. Healthcare resource utilization (including dialysis utilization, number of emergency department [ED] visits, rheumatologist and nephrologist visits, and number of hospitalizations) was based on inpatient and outpatient claims.
Time to Switching Between or Re-initiating Induction Immunosuppressant Therapy
The time to switching between or re-initiating induction immunosuppressant therapy was assessed for the induction and maintenance cohorts, respectively, during the follow-up in which patients were hypothesized to be receiving their treatment. The use of immunosuppressants of interest was identified using pharmacy claims or procedure codes. Patients were followed from the index date until the earliest of any of the criteria described under “Study Design and Data Source”, or a switch between or re-initiation of induction therapy.
The event date of a switch in or re-initiation of induction therapy was set to the earliest of the following: initiation of MMF with pulse corticosteroids or CYC with pulse corticosteroids; switching from MMF with pulse corticosteroids to CYC with pulse corticosteroids and vice versa; or initiation of rituximab, tacrolimus, or cyclosporine. Further details regarding the definition of switching or re-initiating MMF with pulse corticosteroids are shown in the Supplementary Materials.
Time to Conversion to Maintenance Immunosuppressant Therapy
Time to conversion to maintenance immunosuppressant therapy was evaluated among patients in the induction immunosuppressant cohort. Patients were followed from the index date until the earliest of any of the criteria described under “Study Design and Data Source”; or conversion to maintenance immunosuppressant therapy, or a switch to a different induction immunosuppressant therapy. The endpoints of this outcome were conversion to MMF or AZA during follow-up, with the date set to the earliest prescription for either of these medications during follow-up. Specific details regarding the definition of converting to MMF maintenance therapy are shown in the Supplementary Materials.
Corticosteroid Treatment Patterns
The presence of oral or pulse corticosteroids, and mean dose of oral corticosteroids, were assessed in both cohorts based on pharmacy claims at baseline (specifically, the month preceding the index date) and during each month of follow-up that a patient was hypothesized to be using the initiated immunosuppressant (see “Study Design And Data Source”). Patients were subcategorized by mean daily prednisone equivalent doses of ≥ 5 mg/day, ≥ 7.5 mg/day or ≥ 10 mg/day, these subcategories were not mutually exclusive. The mean daily dose of corticosteroids was considered high if the prednisone equivalent dose was ≥ 7.5 mg/day.
The follow-up period for this outcome was the same as the time to switching/re-initiating induction immunosuppressant therapy outcome.
Statistical Analysis
All analyses were descriptive. For baseline characteristics, categorical variables were described using frequencies and percentages; continuous variables were described using mean (standard deviation [SD]) or median (interquartile range [IQR]).
Time-to-event analysis was used to estimate the cumulative incidence and 95% confidence intervals (CI) of either switching or re-initiating induction therapy, and converting to maintenance therapy. Cumulative incidences were calculated for the total induction and maintenance cohorts, and for specific immunosuppressants initiated. Cumulative incidence was estimated at 6 and 12 months for the induction cohort and 6, 12, and 48 months for the maintenance cohort.
The mean prednisone equivalent dose of corticosteroids was calculated for each month of as-treated follow-up by dividing the sum of the prescribed corticosteroid dose (days of medication supplied multiplied by dosage) during each monthly interval by 30 (number of days). The proportion of patients prescribed mean prednisone equivalent doses of ≥ 5 mg/day, ≥ 7.5 mg/day, or ≥ 10 mg/day were calculated during each month.
Results
Demographic and Clinical Characteristics
A total of 5000 patients were included in the study contributing a collective total of 5516 eligible immunosuppressant treatment episodes. Baseline characteristics for patients included in the induction and maintenance immunosuppressant cohorts are summarized in Table 1. Most patients in both cohorts were female, with a mean (SD) age of 38.5 (13.2) years in the induction immunosuppressant cohort and 41.6 (12.6) years in the maintenance immunosuppressant cohort.
A large proportion of patients in both cohorts had an SLE-related condition of interest, including arthritis/arthralgia (induction, 53.0%; maintenance, 45.6%), rash (induction, 36.3%; maintenance, 32.0%) and myalgia/myositis (induction, 15.3%; maintenance, 11.4%). With regards to prior SLE-related medication use, a high proportion of patients in both cohorts had used antimalarials, immunosuppressants, and/or oral corticosteroids in the 12 months preceding the index date (Table 1). The median (IQR) prednisone equivalent doses of oral corticosteroids at baseline were 5.1 (1.2–11.4) mg/day and 3.5 (0.3–8.8) mg/day for the induction and maintenance immunosuppressant cohorts, respectively (Table 1).
The proportion of patients requiring hospitalization (for any reason) during the 12 months preceding the index date was higher in the induction immunosuppressant cohort compared with the maintenance immunosuppressant cohort (induction, 52.4%; maintenance, 38.6%); the highest proportion was observed among patients initiating CYC with pulse corticosteroids (61.1%). Similar proportions of patients in both cohorts required ≥ 1 visit to a rheumatologist or nephrologist (Table 1).
Switching Between or Re-initiating Induction Immunosuppressant Therapy
Among the 372 treatment episodes in the induction immunosuppressant cohort, there were 80 observed switches to an alternative induction immunosuppressant treatment within 12 months of initiation during 178.0 person-years of follow-up (Table 2). The cumulative incidence (95% CI) of induction treatment switching at 12 months among the total cohort was 24.6% (20.1–30.0). Among patients initiating MMF with pulse corticosteroids and those initiating CYC with pulse corticosteroids, the cumulative incidences at 12 months were 24.1% (18.2–31.5) and 25.4% (18.9–33.8), respectively (Table 2). The cumulative incidence of switching at 6 months was 22.4% (18.3–27.4) in the total cohort, 23.1% (17.4–30.2) in the MMF with pulse corticosteroids cohort and 21.7% (16.0–29.1) in the CYC with pulse corticosteroids cohort (Table 2).
Among the 5144 treatment episodes in the maintenance immunosuppressant cohort, there were 1023 re-initiations of induction immunosuppressant therapy within 48 months of treatment initiation during 4291.7 person-years of follow-up (Table 2). The cumulative incidence of re-initiating induction immunosuppressant therapy at 48 months among the total cohort was 31.1% (28.6–33.9). The cumulative incidence of re-initiation was higher among patients that initiated MMF (33.9% [31.0–37.0]) than those that initiated AZA (21.7% [16.7–27.8]; Table 2). At 12 months, the cumulative incidence of re-initiation of induction immunosuppressant therapy were 21.2% (19.9–22.5), 23.4% (22.0–25.0), and 14.1% (12.0–16.7) in the total, MMF and AZA cohorts, respectively (Table 2). The cumulative incidence at 6 months is shown in Table 2. The specific induction immunosuppressants that were switched to or re-initiated are shown in Supplementary Table S1.
Converting to Maintenance Immunosuppressant Therapy
Among the 372 treatment episodes in the induction immunosuppressant cohort, there were 138 conversions to maintenance immunosuppressant therapy within 12 months of treatment initiation during 144.2 person-years of follow-up (Table 3). At 12 months, the cumulative incidence (95% CI) among the total cohort was 59.6% (52.4–66.1). The cumulative incidence was similar between those initiating MMF with pulse corticosteroids (61.1% [51.6–69.3]) and those initiating CYC with pulse corticosteroids (57.0% [46.3–66.2]) (Table 3). More patients converted to MMF maintenance immunosuppressant therapy than AZA, regardless of whether they received MMF or CYC induction (Supplementary Table S2).
Corticosteroids Treatment Patterns
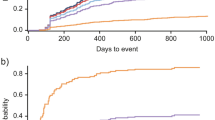
Use of oral corticosteroids among patients in the induction immunosuppressant cohort gradually decreased during follow-up but remained high with 50.8% of patients still using oral corticosteroids of any dose at 12 months; use of pulse corticosteroids also gradually decreased among these patients (Fig. 1A). Trends in oral corticosteroid use appeared to be similar between the induction therapies. Pulse corticosteroid use during follow-up appeared to be lower among patients initiating MMF compared with CYC. Additionally, the proportion of patients receiving high mean prednisone equivalent doses (≥ 7.5 mg/day) of oral corticosteroids decreased during follow-up among patients in the induction immunosuppressant cohort, but 21.5% remained on a high dose at 12 months of follow-up (Fig. 1B).
Proportion of patients with LN in the induction immunosuppressant cohort who were A prescribed oral or pulse corticosteroids during follow-upa and B were receiving respective mean prednisone equivalent doses of oral corticosteroids during follow-up. aThe proportion of patients receiving pulse corticosteroids does not equal 100% for treatment episodes of MMF or CYC with pulse corticosteroids because corticosteroid use for these treatment episodes was assessed in the 14 days prior or following the prescription date for each immunosuppressant; these data report corticosteroid use in the follow-up period. CYC cyclophosphamide, LN lupus nephritis, MMF mycophenolate mofetil
Among patients in the maintenance immunosuppressant cohort, use of oral corticosteroids at any dose gradually decreased from 61.4% in the first month of follow-up to 44.1% at 24 months, when their use appeared to stabilize; use of pulse corticosteroids was low throughout follow-up (Fig. 2A). These trends were observed for patients initiating maintenance therapy with either MMF or AZA. Trends in the percentage of patients prescribed high mean daily prednisone equivalent doses (≥ 7.5 mg/day) of oral corticosteroids also decreased during follow-up for patients initiating maintenance therapy with either MMF or AZA; for both treatments, 15.8% of patients remained on high doses at 24 months of follow-up (Fig. 2B).
Proportion of patients with LN in the maintenance immunosuppressant cohort who were A prescribed oral or pulse corticosteroids during follow-up and B were receiving respective mean prednisone equivalent doses of oral corticosteroids during follow-up. aPatients with MMF treatment episodes for the maintenance immunosuppressant cohort must have had no pulse corticosteroids within 14 days of MMF prescription if switching from CYC induction immunosuppressant therapy; and must have been receiving MMF for 6 months with no pulse corticosteroid use for ≥ 3 months if switching from MMF induction immunosuppressant therapy. bPatients with AZA treatment episodes could be prescribed with or without pulse corticosteroids. AZA azathioprine, LN lupus nephritis, MMF mycophenolate mofetil
Discussion
Treatment switching is recommended when patients with LN become unresponsive or are intolerant to treatment [2, 3]. Several studies have shown that many patients fail to achieve remission, or relapse-free remission when using immunosuppressants with corticosteroids [5, 6, 8]; suggesting that many patients will switch treatment during their disease course. However, no previous study has investigated the real-world treatment patterns of patients with LN who initiated immunosuppressant therapy.
Using US claims-based data from 2012 to 2019, we have shown that around one-fifth of patients with LN initiating either induction or maintenance therapy required a switch in induction immunosuppressant therapy within 6 months of treatment initiation, with incidence rates rising in both cohorts at 12 months. While this study could not capture reasons for switching immunosuppressants during induction therapy, these findings suggest that patients may be either intolerant to current treatments, or treatments may be inadequate at inducing and maintaining renal remission.
Although these data showed that over half of patients who initiated induction immunosuppressant therapy were able to convert to maintenance immunosuppressant therapy within 12 months, around a fifth of patients initiating maintenance immunosuppressant therapy subsequently required re-initiation of induction therapy. This indicates that these patients may not have fully responded to the initial induction therapy, highlighting the unmet needs within the current treatment paradigm for patients with LN. The cumulative incidence of re-initiating induction immunosuppressant therapy was higher in patients who initiated maintenance treatment with MMF compared with AZA. This observation opposes the findings of the Aspreva Lupus Management Study, which showed that MMF was superior to AZA in time to treatment failure, time to renal flare and rescue therapy [21]. However, the present study utilizes real-world data and patients treated with AZA may have had less severe disease and would therefore be less likely to relapse. Additionally, these data are descriptive and so direct comparisons between groups should not be made.
Overall, there were substantially more treatment episodes for the maintenance immunosuppressant cohort than there were for the induction immunosuppressant cohort. This may be because the algorithm used here to define patients with LN may not have identified all cases of induction therapy, due to the inclusion requirement of the use of pulse corticosteroids within 14 days before or after the index date. Alternatively, there may have been a lower incidence of induction treatment as prescribing physicians likely balance potential treatment toxicity with disease severity, suggesting that there could be an unmet need for safe and effective treatments that induce renal remission among patients with active LN.
Reducing corticosteroids use is an important goal of treatment, given the association between sustained use of corticosteroids with organ damage and adverse events [17,18,19]. In this study, oral corticosteroid use declined following initiation of induction and maintenance immunosuppressant therapy, but overall use remained high in the induction immunosuppressant cohort. Similar patterns of corticosteroid use during follow-up were observed among patients initiating MMF and CYC induction treatments. These findings further highlight the need for effective treatments that spare corticosteroid use in the current treatment paradigm. Novel treatments, such as belimumab and voclosporin, have been shown to significantly improve renal response over placebo, while tapering the dose of corticosteroids among patients with LN [22, 23]; the definition of renal response in the belimumab trial prohibited patients from requiring rescue corticosteroid treatment for LN for 80 weeks following tapering of corticosteroid dose to ≤ 10 mg/day [22]. Additionally, in a pooled analysis of real-world observational studies, belimumab was shown to reduce the need for oral corticosteroids after 6 months of treatment among patients with SLE [24].
The patients with LN initiating induction or maintenance immunosuppressant therapy in this study had significant healthcare burden; many had extensive SLE-related conditions, medication use and healthcare utilization in the year preceding initiation of the index immunosuppressant, with around 10% requiring a dialysis event. Additionally, the extent of hospitalizations and ED visits observed at baseline in the current study exceed those reported previously among patients with LN and commercial insurance over a 12-month period [25]. These observations demonstrate the severity of LN among patients requiring induction or maintenance immunosuppressive therapy and suggests that these patients may benefit from new treatments that can better manage their underlying disease.
This study had several limitations that are common in claims-based studies and should be considered when interpreting results. The database was limited by the amount of clinical information it contained. For example, biopsy results were not available, and it was not possible to determine the specific treatment regimens prescribed (e.g., differing treatment regimens for cyclophosphamide). Operational definitions of LN and other conditions were used based on diagnosis codes recorded in claims data; this may have resulted in misclassification as certain conditions could have been under- or over-captured. However, for LN, the definition implemented in this study was similar to previously developed and validated claims-based algorithms [26]. Prescription claims for immunosuppressants and corticosteroids during follow-up were used as a proxy for actual use. This could have resulted in bias if patients did not use their medications after filling their prescriptions; however, this risk was likely mitigated by censoring patients when they were hypothesized to have discontinued their immunosuppressants. Additionally, considering the relatively low numbers of patients in the induction cohort compared with the maintenance cohort, this study may have under-captured initiation of induction immunosuppressant therapy. Finally, patients from claims databases may be more likely to have chronic diseases (such as SLE) and so the data may not be generalizable to the wider US population [27].
Conclusions
We found that many patients with LN who initiated new immunosuppressant treatment had extensive healthcare utilization and SLE-related conditions in the year preceding treatment initiation. Many patients who initiated induction or converted to maintenance immunosuppressant therapy switched or re-initiated induction therapies during follow-up. Despite initial decreases in oral corticosteroid use, patients remained on high oral corticosteroid doses during follow-up. Together, these findings suggest that many patients with LN would benefit from additional, more effective, and well-tolerated treatments options.
References
Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford). 2016;55:252–62.
Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–23.
Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64:797–808.
FDA. Highlights of prescribing information (benlysta). 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761043s021lbl.pdf. Accessed Feb 8, 2023.
Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–28.
Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–12.
Cortés-Hernández J, Torres-Salido MT, Medrano AS, Tarrés MV, Ordi-Ros J. Long-term outcomes–mycophenolate mofetil treatment for lupus nephritis with addition of tacrolimus for resistant cases. Nephrol Dial Transplant. 2010;25:3939–48.
Yap DY, Ma MK, Mok MM, Tang CS, Chan TM. Long-term data on corticosteroids and mycophenolate mofetil treatment in lupus nephritis. Rheumatology (Oxford). 2013;52:480–6.
Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol. 2016;68:1432–41.
Mahajan A, Amelio J, Gairy K, et al. Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression. Lupus. 2020;29:1011–20.
Chakravarty EF, Michaud K, Katz R, Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2013;22:238–44.
Coggins PR, Ravdin RG, Eisman SH. Clinical evaluation of a new alkylating agent: cytoxan (cyclophosphamide). Cancer. 1960;13:1254–60.
Mills KA, Chess-Williams R, McDermott C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch Toxicol. 2019;93:3291–303.
Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatol Ther. 2007;20:229–38.
Song GG, Lee YH. Comparison of treatment response and serious infection using tacrolimus, tacrolimus with mycophenolate mofetil, in comparison to cyclophosphamide as induction treatment for lupus nephritis. Int J Clin Pharmacol Ther. 2020;58:550–6.
Feldman CH, Hiraki LT, Winkelmayer WC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67:1577–85.
Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum. 2012;64:4021–8.
Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30:1955–9.
Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford). 2012;51:1145–53.
Johnson EK, Nelson CP. Values and pitfalls of the use of administrative databases for outcomes assessment. J Urol. 2013;190:17–8.
Dooley MA, Jayne D, Ginzler EM, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365:1886–95.
Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–28.
Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397:2070–80.
Collins CE, Cortes-Hernández J, Garcia MA, et al. Real-world effectiveness of belimumab in the treatment of systemic lupus erythematosus: pooled analysis of multi-country data from the OBSErve studies. Rheumatol Ther. 2020;7:949–65.
Bartels-Peculis L, Sharma A, Edwards AM, et al. Treatment patterns and health care costs of lupus nephritis in a United States payer population. Open Access Rheumatol. 2020;12:117–24.
Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19:741–3.
Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford). 2017;56:1945–61.
Acknowledgements
Funding
This study (GSK study 209758) and payment of the Rapid Service Fee was funded by GSK.
Medical Writing, Editorial, and Other Assistance
Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, referencing, and submission of the manuscript) was provided by Robert Bloxham, PhD, of Fishawack Indicia Ltd, part of Fishawack Health, and was funded by GSK. All authors have authorized the manuscript to be submitted by a third party and have approved all statements and declarations.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Jacob N Hunnicutt, Mary Elizabeth Georgiou, Roger A Levy, and Kerry Gairy all contributed to the conception or design of the study. All authors contributed to the data analysis or its interpretation.
Disclosures
Jacob N Hunnicutt, Mary Elizabeth Georgiou, Liyuan Ma, and Roger A Levy are employees of GSK and hold stocks and shares in the company. Kerry Gairy was an employee of GSK at the time of the study and holds stocks and shares in the company (current affiliation: AstraZeneca, Global Market Access and Pricing, Cambridge, UK).
Compliance with Ethics Guidelines
No direct subject contact or primary collection of individual human subject data occurred. Study results omit subject identification; therefore, informed consent, ethics committee or Institutional Review Board approval was not required. The study data are available from IBM MarketScan Commercial Claims and Encounters database, but restrictions apply to the availability of these data, which were used under license for the current study; GSK had permission to access the database.
Data Availability Statement
All data required for interpretation of these results are included in this article. Study data are Individual Human Data not owned by GSK and their use aligns with the ‘purpose of use’ outlined in the source contract and/or the terms and conditions of use of the data source and complies with any specified prohibitions of use.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kerry Gairy: affiliation at the time of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hunnicutt, J.N., Georgiou, M.E., Ma, L. et al. Real-World Immunosuppressant Treatment Patterns for Patients with Lupus Nephritis in the United States. Rheumatol Ther 10, 1305–1318 (2023). https://doi.org/10.1007/s40744-023-00577-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00577-7