Abstract
Introduction
Differences in psoriatic arthritis (PsA) treatment response between sexes for ixekizumab, an interleukin-17A antagonist, are largely unexplored. This analysis used data from randomized clinical trials (RCTs) evaluating ixekizumab to study differences in treatment response between male and female patients with PsA.
Methods
We used pooled data from patients enrolled in SPIRIT-P1 and SPIRIT-P2 (NCT01695239 and NCT02349295, respectively), phase 3 RCTs evaluating ixekizumab every 4 and 2 weeks in patients with active PsA. Subgroups of patients were defined by sex (male, female). Efficacy was measured by the proportion of male and female patients achieving American College of Rheumatology 20%/50%/70% improvement criteria (ACR20/50/70), minimal disease activity or very low disease activity (MDA/VLDA), and Disease Activity Index for Psoriatic Arthritis (DAPSA) scores representing low disease activity (LDA) or remission through week 156. Changes from baseline in components of the above measures were also assessed through week 156.
Results
Compared to male patients at baseline, female patients were older, had higher body mass index and lower C-reactive protein levels, and had worse tender joint count, Health Assessment Questionnaire Disability Index, and Leeds Enthesitis Index scores. Through week 156, female patients in all treatment arms had lower response rates than male patients in all analyzed composite measures (ACR20/50/70; MDA/VLDA; DAPSA LDA/remission), with significant differences observed at multiple timepoints in both ixekizumab treatment arms. Female patients also had smaller numeric changes from baseline in the composite measures’ individual components.
Conclusion
Compared to female patients, male patients had greater response rates in ACR20/50/70, MDA/VLDA, and DAPSA LDA/remission and numerically larger improvements in these measures’ individual components, although clinical significance is unclear. Continued efforts to understand sex differences in treatment response may provide insights that can help optimize clinical decision making.
Trial registration
ClinicalTrials.gov identifiers, NCT01695239 and NCT02349295.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Approximately equal numbers of men and women are affected by psoriatic arthritis (PsA), but female patients have been observed to have reduced treatment response and lower drug retention rates for tumor necrosis factor inhibitors. |
It is important to understand if PsA treatments offer consistent benefits for both male and female patients. |
This study assessed the efficacy of ixekizumab, an antileukin-17A inhibitor, in male and female patients with PsA. |
What was learned from the study? |
Male and female patients with PsA had significant differences in baseline disease characteristics as well as treatment response. |
Understanding sex differences in PsA could improve outcomes in patients with prior inadequate response to treatment. |
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory autoimmune disease that occurs in about 30% of patients with psoriasis [1, 2]. This disease is associated with musculoskeletal manifestations, including peripheral arthritis, axial disease, enthesitis, and dactylitis, as well as skin and nail psoriasis. It negatively impacts quality of life with a burden comparable to that of axial spondyloarthritis and rheumatoid arthritis (RA) [2,3,4,5]. Several observational studies have reported differences in outcomes between male and female patients with PsA, reflecting a similar pattern also observed in the contexts of axial spondyloarthritis and RA [6,7,8]. PsA affects male and female patients in approximately equal numbers, but female patients generally have been found to have reduced response to treatment as well as lower drug retention rates for tumor necrosis factor inhibitors (TNFi) [5, 6, 9].
Because of this apparent sex difference in treatment response, it is important to understand whether an effective therapeutic agent for PsA provides consistent responses in both male and female patients. Identifying which manifestations and aspects of PsA are responsible for sex differences in treatment response may also support targeting therapies to each individual patient. A more complete knowledge of sex differences could change the management of PsA in male and female patients, which could result in improved outcomes for patients who had prior inadequate response to treatment.
Currently, sex and/or gender differences in treatment response are not well-understood for interleukin-17A (IL-17A) antagonists. It is unknown if patterns in response by sex for IL-17A antagonists aligns with other treatments having different mechanisms of action, such as TNFi and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). Most analyses evaluating sex differences in PsA treatment use data from observational studies [6, 10, 11]. In the analysis we report here, we used data from randomized clinical trials (RCTs) to analyze sex differences in responses to treatment with ixekizumab for PsA. RCTs allocate the study drug in a randomized manner and data are collected in a standardized setting.
Ixekizumab, a high-affinity monoclonal antibody selectively targeting IL-17A, is approved to treat active PsA and has demonstrated superiority to placebo in achievement of American College of Rheumatology 20% improvement criteria (ACR20) in two phase 3 trials of patients with active PsA (SPIRIT-P1 and SPIRIT-P2) [3, 4]. The present study evaluated the efficacy of ixekizumab in male and female patients enrolled in SPIRIT-P1 and SPIRIT-P2 by overall improvement in the signs and symptoms of PsA as measured by the ACR criteria, minimal disease activity (MDA), and Disease Activity Index for Psoriatic Arthritis (DAPSA) scores through to week 156. These analyses also determined the contribution of the individual components of the above measures’ to overall responses observed in male versus female patients with PsA.
Methods
Patients
Full inclusion and exclusion criteria for the SPIRIT-P1 and SPIRIT-P2 RCTs have been reported previously [3, 4]. Briefly, study participants were adults meeting the Classification for Psoriatic Arthritis (CASPAR) criteria for active PsA, with at least 3/68 tender and 3/66 swollen joints at screening and baseline, and active or documented history of plaque psoriasis. Eligible patients in SPIRIT-P1 had no prior use of biologic agents; patients in SPIRIT-P2 had been treated previously with at least one csDMARD and had an inadequate response or intolerance to one or two TNFis. Patients with fibromyalgia or other chronic pain conditions that could confound evaluation were excluded from both the SPIRIT-P1 and SPIRIT-P2 trials [3, 4].
Study Design and the Integrated Database
SPIRIT-P1 and SPIRIT-P2 were randomized, double-blinded, placebo-controlled phase 3 trials; the respective study designs have been published previously [3, 4]. The integrated database used for this analysis is comprised of pooled data from patients in the intent-to-treat population who were randomized to ixekizumab 80 mg every 4 weeks (Q4W), ixekizumab 80 mg every 2 weeks (Q2W), and placebo, during the 24-week, double-blind, placebo-controlled periods of SPIRIT-P1 and SPIRIT-P2. Adalimumab 40 mg was used as an active reference arm in SPIRIT-P1 only; these patients were not included in the integrated database. Both the ixekizumab regimens required a starting dose of 160 mg. Patients who were initially randomized to placebo or adalimumab were re-randomized to ixekizumab Q2W or Q4W for the extension periods from week 24 to week 156.
All patients gave written informed consent before receiving the investigational product or undergoing study procedures. Study protocols were approved by the ethical review board at each participating site prior to patient screening. SPIRIT-P1 and SPIRIT-P2 are registered at ClinicalTrials.gov (NCT01695239 and NCT02349295, respectively) and were conducted in accordance with the standards of the Declaration of Helsinki and Good Clinical Practice Guideline (CPMP/ICH/135/95) [3, 4].
Patient Subgroups and Outcome Measures
Subgroups of patients were defined by sex (male, female). Efficacy was measured for male and female patients as the proportion achieving ≥ 20%, 50%, or 70% improvement from baseline in the ACR criteria (ACR20/50/70), MDA or very low disease activity (VLDA), and DAPSA scores representing low disease activity or remission (DAPSA LDA/remission; score ≤ 14) through week 156.
Changes from baseline in each of the above measures’ components were also measured. The components for ACR criteria include tender joint count (TJC), swollen joint count (SJC), Pain Visual Analog Scale (Pain VAS), Patient’s Global Assessment of Disease Activity (PtGA), Physician’s Global Assessment of Disease Activity (PhGA), Health Assessment Questionnaire Disability Index (HAQ-DI), and C-reactive protein (CRP) level [12]. The components for DAPSA include TJC, SJC, CRP level, PtGA, and Pain VAS [13]. The components for MDA include TJC, SJC, Psoriasis Area Severity Index score (PASI), body surface area affected by plaque psoriasis (BSA), Pain VAS, PtGA, HAQ-DI, and tender enthesial points as measured by the Leeds Enthesial Index (LEI) [14, 15]. BSA as an individual component was not included in these analyses.
Statistical Analyses
Subgroup analyses in the double-blinded treatment period were prespecified for ACR20; all other analyses were post-hoc. Subgroup analyses of male and female patients were performed on the placebo, ixekizumab Q2W, and ixekizumab Q4W treatment groups.
Baseline demographics and disease characteristics were described for male and female patients in each treatment group. P values for comparisons between male and female patients were calculated with analysis of variance for continuous variables and Fisher’s exact test for categorical variables. The Monte Carlo estimate of the exact P value was used for categorical variables with > 2 categories.
The percentage of patients achieving ACR20/50/70, MDA/VLDA, and DAPSA LDA/Remission responses through week 156 were analyzed. P values for comparisons between male and female patients’ responses within the same treatment group were calculated with Fisher’s test. Modified non-responder imputation (mNRI) was used for missing data. For composite endpoints, missing data for each continuous component was imputed first, and then the composite endpoint was derived using the imputed components. The mNRI method was a composite strategy. Missing data that was due to an event related to study drug, such as week 16 inadequate responders, permanent discontinuation due to lack of efficacy, or adverse events, were considered non-responders; all other cases of missing data were imputed using the multiple imputation (MI) method. For MI, intermittent missing data were first imputed using a Markov Chain Monte Carlo method, then the remaining monotone missing data were imputed using a regression model with predictive mean matching on previous data up to the specified patient visit. The imputation was performed by each study (SPIRIT-P1, SPIRIT-P2) and by each treatment (placebo, ixekizumab Q4W, ixekizumab Q2W), and 100 iterations were run (i.e., 100 datasets were generated for each endpoint). SAS PROC MI was the software used for these analyses [16].
Changes from baseline in individual components (TJC, SJC, Pain VAS, PtGA, PhGA, HAQ-DI, CRP, PASI, and LEI) were analyzed through week 156 for male and female patients. Baseline was defined as the last non-missing value on or before the date of first injection of study treatment at week 0. Modified baseline observation carried forward (mBOCF) was used for missing data. The mBOCF method used in these analyses is a hybrid of baseline observation carried forward (BOCF) and last observation carried forward (LOCF). BOCF was applied to patients who discontinued treatment due to adverse events, and LOCF was applied for all other missing data in individual components.
Results
Baseline Characteristics
Of the 679 patients included in the integrated database, 224 were initially randomized to placebo (104 male, 120 female), 229 to ixekizumab Q4W (108 male, 121 female), and 226 to ixekizumab Q2W (98 male, 128 female). Baseline characteristics are summarized in Table 1. The mean age of patients was 51.0 years, and 45.7% of patients were male. Female patients had significantly higher baseline HAQ-DI scores than male patients in all treatment arms (placebo: P < 0.001; ixekizumab Q4W: P = 0.003; ixekizumab Q2W: P = 0.003). Female patients had significantly lower CRP levels at baseline than male patients in the ixekizumab Q2W treatment arm (P = 0.037). Female patients had significantly higher baseline LEI scores than male patients in the placebo and ixekizumab Q4W treatment arms (P = 0.021 and P = 0.031, respectively).
Composite Measures Through Week 156
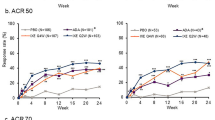
The differences in response rates were significant in ixekizumab-treated patients at multiple timepoints for multiple measures through week 156, and particularly notable significant differences between sexes were observed for ACR50, ACR70, MDA, and DAPSA LDA response rates (Figs. 1, 2). In patients receiving placebo through week 24, female patients had similar response rates compared to male patients in ACR20/50/70, MDA/VLDA, and DAPSA LDA/Remission, with a significantly lower response only observed at week 4 for MDA (Electronic Supplementary Material [ESM] Fig. 1).
Percentage of male and female patients treated with ixekizumab Q4W achieving ACR20/50/70 (a, b), MDA/VLDA (c, d), and DAPSA LDA/DAPSA Remission (e, f) through 156 weeks. Missing data were imputed by mNRI. P values were calculated for proportions of male vs. female responders; *P < 0.05, ‡P < 0.01, †P < 0.001. ACR20/50/70 20%, 50%, or 70% improvement from baseline in the American College of Rheumatology criteria; DAPSA Disease Activity Index for Psoriatic Arthritis, LDA low disease activity, MDA minimal disease activity, Ns number of patients in the subgroup, VLDA very low disease activity, Q4W every 4 weeks
Percentage of male and female patients treated with ixekizumab Q2W achieving ACR20/50/70 (a, b), MDA/VLDA (c, d), and DAPSA LDA/DAPSA Remission (e, f) through 156 weeks. Missing data were imputed by mNRI. P values were calculated for proportions of male vs. female responders; *P < 0.05, ‡P < 0.01, †P < 0.001. DAPSA Disease Activity Index for Psoriatic Arthritis, LDA low disease activity, MDA minimal disease activity, Ns number of patients in the subgroup, VLDA very low disease activity, Q2W every 2 weeks
Changes in Individual Components of Composite Measures through Week 156
In the ixekizumab Q4W treatment arm, female patients had smaller numeric improvements from baseline than male patients in Pain VAS, PtGA, HAQ-DI, CRP, and PASI at multiple timepoints through week 156 (Fig. 3). In the ixekizumab Q2W treatment arm, female patients had smaller numeric improvements from baseline than male patients in Pain VAS, PtGA, HAQ-DI, CRP, and PASI (Fig. 4). The magnitude of the differences in changes from baseline between sexes in ixekizumab-treated patients was greater in HAQ-DI, CRP, and PASI than in Pain VAS and PtGA (Figs. 3, 4). Overall, trends for both ixekizumab treatment arms were similar. In the placebo arm, there were no notable differences in changes from baseline between male and female patients at week 24; statistical significance was not tested (ESM Fig. 2).
Changes from baseline in male and female patients treated with ixekizumab Q4W for TJC (a), SJC (b), Pain VAS (c), PtGA (d), PhGA (e), HAQ-DI (f), CRP (g), PASI (h), and LEI (i) through 156 weeks. Missing data were imputed by mBOCF. TJC includes 68 joints; SJC includes 66 joints. Pain VAS, PtGA, and PhGA are measured on a 0–100 scale. HAQ-DI is measured on a 0–3 scale. CRP is measured in mg/L. PASI is measured on a 0–72 scale. LEI is measured on a 0–6 scale. Change from baseline in PASI was measured in 77 male patients and 64 female patients with ≥ 3% body surface area affected at baseline. Change from baseline in LEI was measured in 57 male patients and 79 female patients with LEI > 0 at baseline. CRP C-reactive protein, HAQ-DI Health Assessment Questionnaire Disability Index, LEI Leeds Enthesitis Index, mBOCF modified baseline observation carried forward, Ns number of patients in subgroup, Pain VAS Pain Visual Analog Scale, PASI Psoriasis Area and Severity Index, PhGA Physician’s Global Assessment of Disease Activity, PtGA Patient’s Global Assessment of Disease Activity, SJC swollen joint count, TJC tender joint count, Q4W every 4 weeks
Changes from baseline in male and female patients treated with ixekizumab Q2W for TJC (a), SJC (b), Pain VAS (c), PtGA (d), PhGA (e), HAQ-DI (f), CRP (g), PASI (h), and LEI (i) through 156 weeks. Missing data were imputed by mBOCF. TJC includes 68 joints; SJC includes 66 joints. Pain VAS, PtGA, and PhGA are measured on a 0–100 scale. HAQ-DI is measured on a 0–3 scale. CRP is measured in milligrams per liter (mg/L). PASI is measured on a 0–72 scale. LEI is measured on a 0–6 scale. Change from baseline in PASI was measured in 67 male patients and 60 female patients with ≥ 3% body surface area affected at baseline. Change from baseline in LEI was measured in 50 male patients and 91 female patients with LEI > 0 at baseline. CRP C-reactive protein, HAQ-DI Health Assessment Questionnaire Disability Index, LEI Leeds Enthesitis Index, mBOCF modified baseline observation carried forward, Ns number of patients in subgroup, Pain VAS Pain Visual Analog Scale, PASI Psoriasis Area and Severity Index, PhGA Physician’s Global Assessment of Disease Activity, PsA psoriatic arthritis, PtGA Patient’s Global Assessment of Disease Activity, SJC swollen joint count, TJC tender joint count, Q2W every 2 weeks
Discussion
We report significant differences between male and female patients with PsA participating in ixekizumab trials with respect to their baseline characteristics and treatment response. At baseline, female patients were older, had higher body mass index (BMI), and had worse TJC, HAQ-DI, and LEI scores with lower CRP levels than male patients. Through week 156, female patients in both ixekizumab treatment arms (Q4W and Q2W) had lower response rates in the composite measures ACR20/50/70, MDA/VLDA, and DAPSA LDA/Remission than male patients, with significant differences observed at multiple timepoints. In the ixekizumab treatment arms, female patients also generally had smaller magnitudes of improvements from baseline than male patients in Pain VAS, PtGA, HAQ-DI, CRP, and PASI.
Differences in improvements of individual components (i.e., Pain VAS, PtGA, HAQ-DI, CRP, and PASI) impact response rates for composite measures (i.e., ACR20/50/70, MDA/VLDA, and DAPSA LDA/Remission). In these analyses, female patients treated with ixekizumab reported less improvement in patient-reported outcomes (PROs) than their male counterparts, particularly for pain, PtGA, and HAQ-DI. However, changes from baseline in physician-assessed outcomes such as TJC, SJC, and PhGA were similar between male and female patients. The differences between sexes in improvement of CRP levels and PASI scores show a response gap in objective markers of inflammation and psoriasis severity, respectively. This may be driven partially by lower baseline CRP levels and PASI scores in female patients, which would then lead to an absolute lower reduction in these scores. There may also be a difference in response between sexes that may be better tracked through PROs than physician-assessed outcomes. When evaluating treatment effect, it is important to consider not only objective, physician-reported assessments of disease severity but also the severity of symptoms reported by patients.
Similar sex disparities were reported in previous observational studies in PsA. In a 2013 study, male patients had better function and quality of life based on several PROs compared to female patients [5]. Female patients have also been reported to have overall worse outcomes in composite measures and individual components (except for TJC and SJC), with significantly higher HAQ-DI scores compared to male patients [17]. A 2020 observational study conducted in 14 countries reported that PROs were worse in female patients than in male patients and that female patients were less likely to meet treatment targets as treat-to-target components (TJC, Pain VAS, HAQ-DI, and PtGA) were harder to achieve in female versus male patients [18].
Several studies support that men with PsA are more likely to develop axial involvement and radiographically visible joint damage, while women with PsA are more likely to experience worse peripheral disease as well as report limited function and worse quality of life [5, 9, 19,20,21,22]. Differences in PsA characteristics appear to be unrelated to human leukocyte antigen B27 (HLA-B27) status [20]. In early PsA, male sex has been found to be a predictor of favorable outcomes at 5 years [17]. Adjusting for baseline risk factors did not impact the association between greater TNFi effectiveness and male sex in a 2018 study, which suggests a potential biological role in the differences in treatment response between sexes or differences in other unmeasured gender-related (sociocultural) factors [11].
Several recent large studies have shown that women tended to discontinue TNFi agents earlier than males. Glintborg et al. analyzed information on 764 patients with PsA treated with TNFi agents from the Danish biologics registry DANBIO [23]. These authors reported that female sex was associated with shorter drug survival with a hazard ratio of 1.4 [23]. This finding could be explained by inferior response to treatment. However, female sex was also associated with shorter drug survival in a stratified model that only included adverse effects as the event causing drug termination, suggesting that women also experienced more side effects that led to TNFi discontinuation [23].
A 2016 systematic literature review of nine articles by Generali et al. found that women had shorter treatment duration with TNFi than men but that retention rates for methotrexate, analyzed only in one of the studies, were similar between men and women [6]. A 2017 systematic literature review and meta regression by Druyts et al. found that ACR50 and PASI 75 responses were lower in trials evaluating biologic treatments that enrolled fewer men [24]. A real-world analysis of patients with PsA using data from the Corrona registry showed patients who discontinued a new TNFi treatment by the first follow-up visit were more likely to be women with higher disease activity and worse PROs than patients who continued with the defined index treatment; however, the difference between men and women was only significant among TNFi-experienced patients [25]. In a prospective observational study of 108 patients treated with biological therapy (93% TNFi and 7% IL-17i), male sex was associated with a higher rate of response to biological treatment in both axial PsA and peripheral PsA; however; in a real-world setting, secukinumab improved disease activity and depressed mood in both men and women with PsA, although women demonstrated an overall higher disease burden [26, 27].
A similar trend was observed in patients with axial spondyloarthritis and RA [28,29,30,31,32]. Therefore, this phenomenon may not be unique to PsA. While the reason for these sex/gender differences in drug survival and response to therapy remains unclear, sex hormones and musculoskeletal performance may explain the poor response to TNFi in women.
Factors that may impact sex differences in PsA may be discerned from this study’s analyses of responses to composite measures and their individual components. Differences in weight and BMI may impact function (as measured by HAQ-DI), Pain VAS, and PtGA; these differences in BMI may also affect drug pharmacokinetics, which may also explain these differences. Function may be more impacted by other conditions, such as obesity, age, comorbidities, and, in women, postmenopausal status. In addition, female patients in these analyses were older at baseline; postmenopausal women are more likely to experience joint symptoms, which may also impact Pain VAS and PtGA. Healthcare providers may also be more likely to downplay the complaints of female patients, which could lead to undertreatment or bias in physician-assessed outcomes [33].
Other possible mechanisms for sex differences in PsA include sex hormones and environmental factors, such as trauma and smoking [9]. Female sex has been associated with a quicker worsening of disability as measured by HAQ-DI and was predictive of worse HAQ-DI scores a decade after PsA onset [9]. Female patients have been reported to be more likely to receive treatment with csDMARDs, which could result in less radiographic damage over time, although the preventative role of treatment with csDMARDs is under debate [5]. However, functional limitations and worse quality of life do not directly reflect inflammation or joint damage. Female patients may have more sensitivity in pain perception, which could explain greater disability [5]. While the present study of patients with PsA used data from trials that excluded patients with physician-recognized fibromyalgia, others have noted the impact of fibromyalgia on outcomes in patients with PsA; a 2020 observational study assessing patients with PsA and treated with TNFi found that female patients with both PsA and fibromyalgia had lower TNFi drug survival and worse TJC, DAPSA, and HAQ scores [34]. Male patients have greater muscle strength, which may make performing daily tasks easier. Muscle strength affects HAQ-DI scores, and, in the context of RA, male patients underemphasize their disability [5]. Muscle mass and strength may lead to more resilience after improvement in joint swelling.
This analysis was limited in that it was conducted post hoc and not powered to assess responses by sex. No statistical tests in the analysis were controlled for type-1 errors, and analyses were not adjusted for the differences between men and women in baseline age, BMI, TJC, HAQ-DI score, LEI score, and CRP levels. Additionally, individual components of the composite measures are affected by multiple factors. For example, CRP levels can be impacted by patients’ race and socioeconomic factors, BMI, other comorbidities, and hormone replacement therapy [35, 36].
The strengths of these analyses include the use of data from phase 3 RCTs, which are standardized. Data were from both bDMARD-naïve and TNFi-experienced patients. These analyses also assessed responses to composite endpoints (ACR20/50/70, MDA/VLDA, and DAPSA LDA/Remission) that have been validated for use in measuring the signs and symptoms of PsA. While treatment with ixekizumab lessened clinical symptoms in both male and female patients, the lower response rates of women can inform clinicians when making treatment decisions. Closer monitoring and follow-up may be warranted for women with PsA. Further studies are needed to better determine different approaches to PsA treatment management in male versus female patients.
Conclusion
This study found that male patients had greater response rates in ACR20/50/70, MDA/VLDA, and DAPSA LDA/Remission as well as greater improvements in the individual components of these measures compared to female patients. While composite measures are useful in evaluating treatment response because they assess multiple domains of PsA, male and female patients may perceive or report PsA disease severity differently, which may affect individual components of these composite measures, such as Pain VAS, PtGA, and HAQ-DI. Overall, differing response rates in composite measures ultimately reflects the impact of differences among the individual components of these measures. A more complete knowledge of sex and gender differences in PsA could result in improved outcomes for difficult-to-treat patients with prior inadequate response to treatment.
References
Gladman DD. Psoriatic arthritis. Dermatol Ther. 2009;22(1):40–55.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–70.
Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87.
Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086):2317–27.
Eder L, Thavaneswaran A, Chandran V, Gladman DD. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis. 2013;72(4):578–82.
Generali E, Sciré CA, Cantarini L, Selmi C. Sex differences in the treatment of psoriatic arthritis: a systematic literature review. Israel Med Assoc J. 2016;18(3–4):203–8.
Wright GC, Kaine J, Deodhar A. Understanding differences between men and women with axial spondyloarthritis. Semin Arthritis Rheum. 2020;50(4):687–94.
Hekmat K, Jacobsson LT, Nilsson J, Lindroth Y, Turesson C. Changes and sex differences in patient reported outcomes in rheumatoid factor positive RA-results from a community based study. BMC Musculoskelet Disord. 2014;15:44.
Eder L, Gladman DD. Predictors for clinical outcome in psoriatic arthritis—What have we learned from cohort studies? Expert Rev Clin Immunol. 2014;10(6):763–70.
Colombo D, Chimenti S, Grossi PA, et al. Prevalence of acute and chronic viral seropositivity and characteristics of disease in patients with psoriatic arthritis treated with cyclosporine: a post hoc analysis from a sex point of view on the observational study of infectious events in psoriasis complicated by active psoriatic arthritis. Clin Cosmet Investig Dermatol. 2016;9:1–7.
Højgaard P, Ballegaard C, Cordtz R, et al. Gender differences in biologic treatment outcomes-a study of 1750 patients with psoriatic arthritis using danish health care registers. Rheumatology (Oxford). 2018;57(9):1651–60.
Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729–40.
Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis. 2010;69(8):1441–7.
Taylor PC, Lee YC, Fleischmann R, et al. Achieving pain control in Rheumatoid Arthritis with Baricitinib or Adalimumab plus Methotrexate: results from the RA-BEAM Trial. J Clin Med. 2019;8(6):831.
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53.
Yuan Y. Multiple imputations using SAS software. J Stat Softw. 2011;45(6):1–25.
Theander E, Husmark T, Alenius GM, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis. 2014;73(2):407–13.
Orbai AM, Perin J, Gorlier C, et al. Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with sex in 458 patients from fourteen countries. Arthritis Care Res. 2020;72(12):1772–9.
Queiro R, Sarasqueta C, Torre JC, Tinturé T, López-Lagunas I. Comparative analysis of psoriatic spondyloarthropathy between men and women. Rheumatol Int. 2001;21(2):66–8.
Gladman DD, Brubacher B, Buskila D, Langevitz P, Farewell VT. Psoriatic spondyloarthropathy in men and women: a clinical, radiographic, and HLA study. Clin Invest Med. 1992;15(4):371–5.
Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—an analysis of 220 patients. Q J Med. 1987;62(238):127–41.
Duruöz MT, Gezer HH, Nas K, et al. Gender-related differences in disease activity and clinical features in patients with peripheral psoriatic arthritis: A multi-center study. Joint Bone Spine. 2021;88(4):105177.
Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63(2):382–90.
Druyts E, Palmer JB, Balijepalli C, et al. Treatment modifying factors of biologics for psoriatic arthritis: a systematic review and Bayesian meta-regression. Clin Exp Rheumatol. 2017;35(4):681–8.
Mease PJ, Karki C, Liu M, et al. Discontinuation and switching patterns of tumour necrosis factor inhibitors (TNFis) in TNFi-naive and TNFi-experienced patients with psoriatic arthritis: an observational study from the US-based Corrona registry. RMD Open. 2019;5(1):e000880.
Benavent D, Plasencia C, Navarro-Compán V, et al. AB0736 Gender influence on treatment effectiveness in psoriatic arthritis. Ann Rheum Dis. 2019;78:1832–3.
Kiltz U, Brandt-Juergens J, Kästner P, Riechers E, Peterlik D, Tony HP. POS1023 How does gender affect secukinumab treatment outcomes and retention rates in patients with psoriatic arthritis?—real world data from the german aquila study. Ann Rheum Dis. 2021;80:780–1.
Markenson JA, Gibofsky A, Palmer WR, et al. Persistence with anti-tumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol. 2011;38(7):1273–81.
Heiberg MS, Koldingsnes W, Mikkelsen K, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008;59(2):234–40.
Pavelka K, Forejtová S, Stolfa J, et al. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol. 2009;27(6):958–63.
Neuenschwander R, Hebeisen M, Micheroli R, et al. Differences between men and women with nonradiographic axial spondyloarthritis: clinical characteristics and treatment effectiveness in a real-life prospective cohort. Arthritis Res Ther. 2020;22(1):233.
Micheroli R, Tellenbach C, Scherer A, et al. Effectiveness of secukinumab versus an alternative TNF inhibitor in patients with axial spondyloarthritis previously exposed to TNF inhibitors in the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2020;79(9):1203–9.
Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med. 2012;10:82.
Iannone F, Nivuori M, Fornaro M, Venerito V, Cacciapaglia F, Lopalco G. Comorbid fibromyalgia impairs the effectiveness of biologic drugs in patients with psoriatic arthritis. Rheumatology (Oxford). 2020;59(7):1599–606.
Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212.
Bray C, Bell LN, Liang H, et al. Erythrocyte sedimentation rate and c-reactive protein measurements and their relevance in clinical medicine. WMJ. 2016;115(6):317–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies and the Rapid Service Fee were sponsored by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Lihi Eder contributed to the conception of the work, design of the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. Hans-Peter Tony contributed to the acquisition of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. Satish Odhav, Trev Sprabery, Amanda Gellett, Clint Bertram, and Alexis Ogdie contributed to the interpretation of data for the work and critical revision of the manuscript for important intellectual content. Eva Galindez Agirregoikoa contributed to the analysis of data for the work, interpretation of data for the work, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Marius Korkosz contributed to the acquisition of data of the work and critical revision of the manuscript for important intellectual content. Sergio Schwartzman contributed to the analysis of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. So Young Park contributed to the analysis of data for the work, interpretation of data for the work, and drafting of the manuscript
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Nicole Lipitz of Syneos Health for medical writing support, funded by Eli Lilly and Company.
Disclosures
Lihi Eder has received unrestricted educational and research grants and consulting fees from Abbvie, Amgen, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Sandoz, and UCB. Hans-Peter Tony has received payments from Eli Lilly and Company. Satish Odhav and/or his institution has received research grants and speaking or consulting fees from Abbvie, Amgen, Astra Zeneca, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Exagen, Galapagos (Gilead), GSK, Horizon, Ironwood, Janssen, Mallinckrodt, Nektar, Nichi-Iko, Novartis, Pfizer, Primus, Regeneron, Sanofi, Selecta, and UCB. Eva Galindez Agirregoikoa and Mariusz Korkosz have no payments to disclose. Sergio Schwartzman has received speaking fees from Abbvie, Genentech, Janssen, Eli Lilly and Company, Novartis, Pfizer, Regeneron, Sanofi, UCB; has stock ownership for Amgen, Boston Scientific, Gilead, Medtronic, and Pfizer; has received consulting fees from Abbvie, Crescendo, Dermtech, Janssen, Gilead, Lilly, Myriad, Novartis, Regeneron, Samsung, Sanofi, and UCB; serves as a board member of the National Psoriasis Foundation; and serves on the scientific advisory board for Myriad. Aubrey Trevelin Sprabery, Amanda M Gellett, Chen-Yen Lin, So Young Park, and Clinton C Bertram report employment with and stock ownership for Eli Lilly and Company. At the time of this publication, Amanda M Gellett is now affiliated with Sanofi Genzyme. Alexis Ogdie and/or her institution has received grants from Amgen, Novartis, and Pfizer; has received consulting fees from Abbvie, Amgen, Bristol Myers Squibb, Celgene, Corrona, Eli Lilly and Company, Pfizer, and Novartis; has received speaking fees from Abbvie, Amgen, and Celgene; and has received royalties from Novartis.
Compliance with Ethics Guidelines
The SPIRIT-P1 and SPIRIT-P2 trials were conducted in accordance with the ethical principles of the Declaration of Helsinki as well as local laws and regulations. All trial participants provided written informed consent. Protocols and consent forms were approved by the institutional review board or ethics committee of each site, including the Western Institutional Review Board (SPIRIT-P1) and the Bellberry Human Research Ethics Committee (SPIRIT-P2). A listing of individual sites for the trials are included in the supplements of the primary manuscripts [3, 4]. Both trials were registered on ClinicalTrials.gov (SPIRIT-P1: NCT01695239, SPIRIT-P2: NCT02349295).
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Prior Presentation
An overview of preliminary results on PsA patient subgroups related to this post-hoc analysis was presented at the 2019 Annual Scientific Congress of the European League Against Rheumatism (EULAR) from June 12 through 15 in Madrid, Spain.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eder, L., Tony, HP., Odhav, S. et al. Responses to Ixekizumab in Male and Female Patients with Psoriatic Arthritis: Results from Two Randomized, Phase 3 Clinical Trials. Rheumatol Ther 9, 919–933 (2022). https://doi.org/10.1007/s40744-022-00445-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00445-w