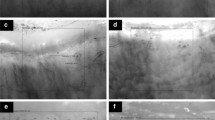
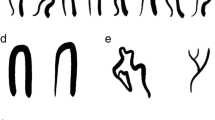Abstract
Purpose of Review
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by vascular complications, endothelial cell injury with subsequent dysfunction. Nailfold video capillaroscopy (NVC) is a non-invasive and reliable point-of-care method that can be used to directly visualize microscopic digital capillaries and monitor patients. In this review, we will describe NVC’s role in assessing SLE disease activity and prognosticating risk for developing vascular complications.
Recent Findings
In SLE, qualitative NVC changes like the presence of microhemorrhages and enlarged capillaries correlate with disease activity. These abnormalities are also associated with specific autoantibody profiles (e.g., anti-phospholipid and anti-U1-RNP antibodies). Recent studies show NVC changes may increase risks of digital ulceration, pulmonary arterial hypertension, and accelerated cardiovascular disease.
Summary
NVC may be used in personalized medicine as an adjunct in assessing SLE activity which may help prognosticate risk of serious complications, but more studies are needed to further delineate its role in specific SLE disease subtypes.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hermansen ML, et al. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford). 2017;56(5):709–15.
Tektonidou MG, et al. Subclinical atherosclerosis in systemic lupus erythematosus: comparable risk with diabetes mellitus and rheumatoid arthritis. Autoimmun Rev. 2017;16(3):308–12.
Castejon R, et al. Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus. 2016;25(2):129–36.
Rajagopalan S, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103(10):3677–83.
Taraborelli M, et al. Endothelial dysfunction in early systemic lupus erythematosus patients and controls without previous cardiovascular events. Arthritis Care Res. 2018;70(9):1277–83.
Raynaud M. De l’asphyxie locale et de la gangrène symétrique des extrémités. Paris: Leclerc; 1862.
Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis. 2001;60(4):407–9.
Smith V, et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (Oxford). 2016;55(5):883–90 This is an important study that describes the reliability of evaluating capillary changes in rheumatologists with varying expertise.
Gorski S, et al. Microangiopathy in naifold videocapillaroscopy and its relations to sE- selectin, endothelin-1, and hsCRP as putative endothelium dysfunction markers among adolescents with Raynaud’s phenomenon. J Clin Med, 2019: 8(5): 567.
Avouac J, et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum. 2017;47(1):86–94.
Koenig M, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58(12):3902–12. This is a critical study that prospectively evaluated the development of connective tissue diseases in patients with Raynaud’s phenomenon with or without the presence of autoantibodies.
Liu Y, Kaplan MJ. Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30(5):441–8.
Heshmat NM, El-Kerdany TH. Serum levels of vascular endothelial growth factor in children and adolescents with systemic lupus erythematosus. Pediatr Allergy Immunol. 2007;18(4):346–53.
Kuryliszyn-Moskal A, et al. Vascular endothelial growth factor in systemic lupus erythematosus: relationship to disease activity, systemic organ manifestation, and nailfold capillaroscopic abnormalities. Arch Immunol Ther Exp (Warsz). 2007;55(3):179–85.
Aterido A, et al. Genome-wide pathway analysis identifies VEGF pathway association with oral ulceration in systemic lupus erythematosus. Arthritis Res Ther. 2017;19(1):138.
Zhao P, et al. Circulating angiogenic T cells are increased in lupus nephritis patients. Med Sci Monit. 2018;24:5384–90.
Moneib HA, et al. Assessment of serum vascular endothelial growth factor and nail fold capillaroscopy changes in systemic lupus erythematosus with and without cutaneous manifestations. J Dermatol. 2012;39(1):52–7 This is an interesting study which highlighted the correlation between NVC abnormalities and increased VEGF and SLE disease activity.
Icli A, et al. Endocan levels and subclinical atherosclerosis in patients with systemic lupus erythematosus. Angiology. 2016;67(8):749–55.
Clement M, et al. CD4+CXCR3+ T cells and plasmacytoid dendritic cells drive accelerated atherosclerosis associated with systemic lupus erythematosus. J Autoimmun. 2015;63:59–67.
Lee PY, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56(11):3759–69.
Buie JJ, et al. IFN-alpha negatively regulates the expression of endothelial nitric oxide synthase and nitric oxide production: implications for systemic lupus erythematosus. J Immunol. 2017;199(6):1979–88.
Rhoads JP, et al. oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcgammaR cooperation and is dependent on CARD9. J Immunol. 2017;198(5):2105–14.
Pieterse E, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2017;37(7):1371–9.
Rother N, et al. Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis. Front Immunol. 2017;8:1136.
Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(1):202.
Conti F, et al. Subclinical atherosclerosis in systemic lupus erythematosus and antiphospholipid syndrome: focus on beta2GPI-specific T cell response. Arterioscler Thromb Vasc Biol. 2014;34(3):661–8.
Cutolo M, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27(1):155–60 This is one of the seminal descriptions for capillaroscopy abnormalities in various rheumatological conditions including SLE.
Lambova S, Hermann W, Muller-Ladner U. Capillaroscopic pattern at the toes of systemic sclerosis patients: does it “tell” more than those of fingers? J Clin Rheumatol. 2011;17(6):311–4.
Shenavandeh S, Habibi S. Nailfold capillaroscopic changes in patients with systemic lupus erythematosus: correlations with disease activity, skin manifestation and nephritis. Lupus. 2017;26(9):959–66.
Caspary L, et al. Alterations of the nailfold capillary morphology associated with Raynaud phenomenon in patients with systemic lupus erythematosus. J Rheumatol. 1991;18(4):559–66.
Pavlov-Dolijanovic SD. N; Vujasinovic Stupar, NZ; Marcetic, DR; Sefik Bukilica, MN; Petrovic, RR, Is there a difference in systemic lupus erythematosus with and without Raynaud’s phenomenon? Rheumatol Int. 2013;33:859–65.
Kabasakal Y, et al. Quantitative nailfold capillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann Rheum Dis. 1996;55(8):507–12.
Riccieri V, et al. Nailfold capillaroscopy changes in systemic lupus erythematosus: correlations with disease activity and autoantibody profile. Lupus. 2005;14(7):521–5.
Lambova SN, Muller-Ladner U. Capillaroscopic pattern in systemic lupus erythematosus and undifferentiated connective tissue disease: what we still have to learn? Rheumatol Int. 2013;33(3):689–95.
Wu PC, et al. Clinical applicability of quantitative nailfold capillaroscopy in differential diagnosis of connective tissue diseases with Raynaud’s phenomenon. J Formos Med Assoc. 2013;112(8):482–8.
Ohtsuka T. Nailfold capillary abnormalities in patients with Sjogren’s syndrome and systemic lupus erythematosus. Br J Dermatol. 1997;136(1):94–6.
Dancour MA, et al. Nailfold videocapillaroscopy in patients with systemic lupus erythematosus. Rheumatol Int. 2006;26(7):633–7.
Cutolo M, et al. Nailfold capillaroscopy in systemic lupus erythematosus: a systematic review and critical appraisal. Autoimmun Rev. 2018;17(4):344–52 This well-written and comprehensive systematic review discusses some of the important reports describing NVC changes in SLE patients.
Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357(9273):2042–8.
Ragab O, Ashmawy A, Abdo M, Mokbel A. Nailfold capilloroscopy in systemic lupus erythematosus. Egypt Rheumatol. 33:61–7.
Studer A, et al. Quantitative nailfold capillary microscopy in cutaneous and systemic lupus erythematosus and localized and systemic scleroderma. J Am Acad Dermatol. 1991;24(6 Pt 1):941–5.
Furtado RN, et al. Scleroderma-like nailfold capillaroscopic abnormalities are associated with anti-U1-RNP antibodies and Raynaud’s phenomenon in SLE patients. Lupus. 2002;11(1):35–41.
Ingegnoli F, et al. Evaluation of nailfold videocapillaroscopic abnormalities in patients with systemic lupus erythematosus. J Clin Rheumatol. 2005;11(6):295–8.
Ciolkiewicz M, Kuryliszyn-Moskal A, Klimiuk PA. Analysis of correlations between selected endothelial cell activation markers, disease activity, and nailfold capillaroscopy microvascular changes in systemic lupus erythematosus patients. Clin Rheumatol. 2010;29(2):175–80.
Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med. 1990;112(9):682–98.
Bongard O, et al. Association of anticardiolipin antibodies and abnormal nailfold capillaroscopy in patients with systemic lupus erythematosus. Lupus. 1995;4(2):142–4.
Kuryliszyn-Moskal A, et al. Clinical significance of nailfold capillaroscopy in systemic lupus erythematosus: correlation with endothelial cell activation markers and disease activity. Scand J Rheumatol. 2009;38(1):38–45.
Merashli M, Alves J, Ames PRJ. Clinical relevance of antiphospholipid antibodies in systemic sclerosis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2017;46(5):615–24.
ter Borg EJ, et al. Clinical associations of antiribonucleoprotein antibodies in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 1990;20(3):164–73.
Donnarumma JFS, et al. Nailfold capillaroscopy as a risk factor for pulmonary arterial hypertension in systemic lupus erythematosus patients. Adv Rheumatol. 2019;59(1):1 This recent report highlights the importance of the SSc-spectrum pattern in predicting the development of pulmonary arterial hypertension in patients with SLE.
Zhang N, et al. Pulmonary arterial hypertension in systemic lupus erythematosus based on a CSTAR-PAH study: baseline characteristics and risk factors. Int J Rheum Dis. 2019;22(5):921–8.
Rosato E, et al. Digital ulcers as an initial manifestation of systemic lupus erythematosus. Intern Med. 2011;50(7):767–9.
Chung HT, et al. Subclinical deterioration of left ventricular function in patients with juvenile-onset systemic lupus erythematosus. Lupus. 2015;24(3):263–72.
Cavazzana I, et al. Relationship between endothelial dysfunction, videocapillaroscopy and circulating CD3+CD31+CXCR4+ lymphocytes in systemic lupus erythematosus without cardiovascular risk factors. Lupus. 2019;28(2):210–6 The authors in this recent article link the presence of vascular T cells with abnormalities in patients with SLE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shahna Tariq declares that there are no conflicts of interest.
Jan Willem Cohen Tervaert declares that there are no conflicts of interest.
Mohammed Osman declares that there are no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lupus
Rights and permissions
About this article
Cite this article
Tariq, S., Tervaert, J.W.C. & Osman, M. Nailfold Capillaroscopy in Systemic Lupus Erythematosus (SLE): a Point-of-Care Tool That Parallels Disease Activity and Predicts Future Complications. Curr Treat Options in Rheum 5, 336–345 (2019). https://doi.org/10.1007/s40674-019-00133-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-019-00133-x