Abstract
Purpose
Real-world evidence (RWE) with regard to allergen-specific immunotherapy (AIT) adherence is increasingly available. Economic modelling has already shown AIT to be cost-effective in the treatment of allergic rhinitis compared with symptomatic treatment. However, analyzing sublingual (SLIT) and subcutaneous (SCIT) immunotherapeutic approaches based on RWE adherence data are not available for Germany. This analysis outlines the cost-effectiveness of SCIT compared with SLIT as well as a symptomatic treatment modality on the basis of recent RWE adherence data.
Methods
A Markov model, with predefined disease stages and a time period of 9 years, was adapted for this analysis. A 6-grass subcutaneous allergoid SCIT preparation and a 5-grass pollen SLIT tablet was employed as AIT administrations. Quality-adjusted life years (QALYs) were calculated based on symptom scores and used as the effectiveness variable. Total costs and cost effectiveness of SCIT, SLIT and symptomatic treatment (ST) were calculated. Model uncertainties were estimated by means of additional sensitivity analyses. Applied discount rate was 3%.
Results
Both SCIT and SLIT preparations proved superior compared to symptomatic treatment with regard to effectiveness. Although more expensive, AIT also proved to be cost-effective. A direct comparison of SCIT (Allergovit®) and SLIT (Oralair®) showed lower total costs for SCIT treatment over the study period of 9 years (SCIT 1779 € versus SLIT 2438 €) and improved effectiveness (SCIT 7.17 QALYs versus SLIT 7.11 QALYs).
Conclusion
AIT represents a cost-effective treatment option for patients with allergic rhinitis compared with symptomatic treatment. SCIT appeared to be dominant and cost-effective, due in particular to higher patient adherence and lower drug costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Allergic rhinitis (AR) is a common chronic inflammatory disease of the nasal mucosa associated with numerous symptoms (i.e., runny, itchy, or blocked nose and itchy, gritty, or watery eyes). Patients often experience reduced sleep quality, emotional problems, and social difficulties resulting in an impaired quality of life [1]. Furthermore, AR is frequently associated by comorbidities such as asthma or unspecific bronchial hyperreactivity, whereas up to 80% of asthma patients also suffer from AR [2].
AR is provoked by sensitivity to environmental allergens and recommended options for handling and treatment comprise: (1) avoiding contact with allergens, (2) symptom-relieving medications such as oral antihistamines (OAH) and intranasal corticosteroids (INCS), and (3) allergen-specific immunotherapy (AIT) [3].
AIT is a treatment strategy for patients with moderate-to-severe AR that is uncontrolled by antiallergic drugs [3]. It is effective in improving symptoms, reducing symptom-relieving medications and combined symptom and medication scores in patients with AR with evidence suggesting that these benefits persist after discontinuation of therapy [4]. Continuous AIT for a period of at least 3 years (as recommended by international guidelines) may modify the course of the disease and achieve long-term remission of symptoms for several years without further need for AIT treatment [5, 6]. In this, AIT is the only treatment with a disease-modifying effect in IgE-mediated allergic diseases and can deliver long-term clinical benefits that may persist for years after treatment discontinuation [7,8,9]. Subcutaneous immunotherapy (SCIT) has been continuously used for AIT since its first demonstration of efficacy by Noon in 1911 [8]. SCIT is administered by a physician during recurrent office visits, particularly during the induction phase with postinjection observations. Sublingual immunotherapy (SLIT) can be administered at home following the initial supervised office-administered dose, which may be more convenient for certain patients.
Both SCIT and SLIT have demonstrated good clinical efficacy for the management of AR and asthma, and the availability of both formulations offers clinicians and patients a wide choice of treatment options. Since AIT requires repeated administration of the vaccine over a long period of, for example, 3 years in order to achieve optimum clinical effectiveness, adherence is a major problem, from the perspective of patients, providers, and payers [3, 10].
It is well accepted that management of AR patients results in a higher financial burden to patients, healthcare providers, and society [11,12,13].
However, economic studies are based on efficacy data from clinical trials while adherence had to be approximated from few observational studies due to scarce availability of real-world data [14, 15].
In real life settings, adherence to AIT is characterized by widely varying premature discontinuation rates [16,17,18]. Recent results from a real world adherence study of a German longitudinal prescription database provided first comparative real-world evidence on therapy adherence and asthma development in patients receiving SCIT or SLIT for grass and tree pollen-induced AR compared to non-AIT patients [19].
A recent review reported strong evidence that AIT is cost-effective in the management of AR and asthma as compared with standard drug treatment alone [20]. The magnitude of AIT’s cost-effectiveness is likely to be underestimated, as most of the studies considered direct costs during active treatment periods without incorporating long-term benefits or preventive or prophylactic effects.
The objective of the present study was to analyze the cost-effectiveness of different administration routes of AIT compared to symptom-relieving medications in AR patients based on current real-world adherence data for Germany.
Methods
Our modelling framework is adapted from modelling approaches of previously published cost effectiveness analyses (CEA) [15, 21, 22]. Economic comparison was based on a product specific modeling approach, applying a 5-grass pollen sublingual tablet for SLIT and 6‑grass subcutaneous allergoid for SCIT in AR patients.
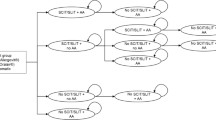
The Markov model is based on predefined disease stages and corresponding transition probabilities for all treatment modalities in order to predict the long-term course of disease in a specific patient cohort (Fig. 1). Treatment alternatives included: SCIT, SLIT, and symptomatic treatment (ST) alone. The Markov model consists of a 1-year cycle length and a time horizon of 9 years. Additional ST was allowed in both AIT arms. AIT duration was assumed to be 3 years.
Patients entered the Markov cycles at a mean age of 29 years, suffering from grass pollen-related AR or rhinoconjunctivitis, but no allergic asthma (AA). During consecutive model cycles, patients were also subject to develop AA resulting with increased mortality and reduced quality of life. The model also assumed that incident asthmatics are suffering during the complete pollen season for the following cycles. A relative risk reduction for both SCIT and SLIT of annual AA incidence of 0.505 was assumed [10, 11]. For those patients who discontinued AIT prior to the end of the 3‑year period, it was assumed that no quality of life changing impact or risk-reducing effects on AA incidence would be seen following AIT discontinuation [23].
The percentage of patients who discontinued AIT prematurely was determined on the basis of the recently published study results by Vogelberg et al. [19]. It was also assumed that patients did not re-initiate AIT following discontinuation. The grass pollen season lasts 4.5 months per year on average in Germany [24].
Costs and use of resources
Costs for all treatment arms of the model were determined from a health insurance perspective. Drug costs for the SCIT 6‑grass allergoid (preseasonal treatment; Allergovit®) were obtained on the basis of the required prescription quantities specified in the professional expert information resulting in seven injections within three quarters of consultation (Table 1; [25]). The number of packs required for the entire treatment period was then calculated according to the time period covered by each pack yielding a total number of three packs necessary for a 3 years’ treatment. According to the professional expert information for the 5‑grass pollen tablet (Oralair®), treatment should be initiated 4 months prior to, and continued throughout the pollen season [26]. According to this information, it was assumed that two packs of 90 tablets each, as well as three packs of 31 tablets each, were required per treatment year. The number of required packs for both drugs was then multiplied by the pharmacy retail price, including value added tax (VAT) and deducting mandatory rebates. AIT drug costs accrued to 1147.41 € for the allergoid and 3123.81 € for the tablet for the entire 3‑year treatment period with costs being distributed equally over the 3 treatment years. For those patients who discontinued AIT, drug costs were reduced by 50% for the year in which treatment was discontinued.
The cost of additional symptomatic treatment (OAH, INCS) was calculated on the basis of costs for loratadine or budesonide. The model also involves the costs for diagnostics, consultations, SCIT injections, and treatment costs upon onset of AA (Table 1). In addition to this, seven injections per year with medical specialists would occur for the SCIT arm over the 3‑year treatment duration. On average this would result in two or three quarterly consultation fees. Hence, conservatively three quarterly fees per year were assumed for patients under SCIT (preseasonal treatment). According to prescribing information for the SLIT tablet patients have consultations over a time period of three quarters [26]. A total of 1.9 contacts with medical specialists in different reimbursement quarters were assumed for all AIT patients for the time following the 3‑year AIT treatment period [5]. Allergy diagnostic work-up was assumed to be performed in the first year in all AIT patients prior to treatment initiation.
Effectiveness parameters
Quality-adjusted life years (QALYs) were selected as primary health outcome measure for therapeutic effectiveness. QALYs integrate quantity-of-life and quality-of-life impacts. Assessment of strength of preference values for patients’ own health, or disease states from a societal perspective result in utilities, ranging from 0 to 1 with 1 representing perfect health. Utility values reflecting impairment to quality of life during the pollen season were applied from Reinhold et al. [21]. Symptoms were assumed not being present outside the pollen season. In addition, the number of new-onset cases of AA was determined based on Shaaban et al. and Verheggen et al. [15, 27].
Model calculation outcomes
Total costs per treatment group, QALYs, and the expected number of AA cases were simulated over the time period of 9 years. Incremental cost-effectiveness ratios (ICER) are reported (i.e., costs per QALY gained) in the case of additional costs—but greater therapeutic benefit—compared with the comparative treatments. All prognosed effects and costs were discounted at a rate of 3% [30].
Sensitivity analysis
Both probabilistic and deterministic sensitivity analyses were performed in order to estimate impact of inaccuracy of assumptions. Probabilistic sensitivity analysis was repeated 1000 times drawing value for all variables at random (from the value ranges listed in Table 2). In contrast, the deterministic sensitivity analysis consecutively varied individual influencing factors in the model with minimum and maximum values and documented the main analysis result after each variation.
Results
Costs
For SCIT, total 9‑year per-patient treatment costs were 1779 €, for SLIT 2438 € and 754 € per symptomatically treated patient (Table 3). The majority of these costs were incurred for AIT drug costs. Based on recently observed real-world AIT discontinuation rates, 486 of the 1000 patients initially treated completed the entire 3‑year treatment period with the SCIT allergoid and 331 with the SLIT tablet. AIT drug costs for patients being fully adherent over 3 years totaled to 1147.41 € for SCIT and 3123.81 € for SLIT. Average 3‑year AIT costs (based on RWE adherence data including patients discontinuing AIT) in Germany accrued to 859 € for SCIT and 1681 € for SLIT. Treatment costs for AA were comparatively modest due to the low AA incidence of 0.46% per year.
Effectiveness
Both AIT groups showed superior QALY effects compared to symptomatic treatment. Patients receiving symptomatic treatment alone accumulated 7.89 (7.04) QALYs (discounted) over the modeling period, whereas SCIT and SLIT achieved 8.04 (7.17) and 7.98 (7.11) QALYs, respectively. While AA occurred in 40 of 1000 patients receiving symptomatic treatment only, the number of incident asthmatics is lower at 26 cases (SCIT) and 31 cases (SLIT). Thus, the reduction in quality of life associated with the presence of AA becomes more relevant in the symptomatic treatment group and results in fewer QALYs.
Cost-effectiveness
SCIT compared to SLIT is economically superior, resulting in a saving of 674 €. Since SCIT was also associated with better effectiveness in terms of QALYs gained as well as with regard to number of incident asthmatics, it is dominant over SLIT and thus cost-effective compared to both SLIT and ST.
A direct comparison of SCIT to symptomatic treatment revealed additional costs of 1042 € for SCIT patients alongside with better effects, both in terms of QALYs gained and the number of new-onset AA cases. Costs per QALY gained (ICER) are 7118 €, thereby putting them in the range considered as cost-effective (according to internationally accepted threshold values of maximally 50,000 € per QALY gained). SLIT also showed better effects compared with purely symptomatic treatment at higher additional costs of 1716 € and lead to a higher cost per QALY of 21,006 €.
Sensitivity analysis
Cost and effectiveness results were robust in probabilistic sensitivity analyses. SCIT compared to SLIT as well as to ST showed superior effectiveness in all cases, both in terms of additional QALYs determined and SCIT-related savings (SCIT vs. SLIT p < 0.001; SCIT vs. ST p < 0.001; Figs. 2 and 3). The deterministic sensitivity analysis identified the variables discontinuation rates, as well as the calculated AIT drug costs as those items causing the greatest degree of uncertainty in terms of difference of costs. With regard to QALYs gained in the treatment groups, the variables assumptions on symptom scores and pollen season duration were most influential.
Discussion
AIT is an effective treatment strategy for patients with moderate-to-severe allergic rhinitis if patients adhere to SCIT or SLIT regimes for more than two continuous seasonal cycles [23, 36]. Nonpersistence to AIT is associated with high costs and poor clinical outcomes [36, 37]. Hence, adherence to treatment is essential for improving the long-term effectiveness of treatment in patients with allergic respiratory diseases, reducing healthcare costs, and for minimizing the disease’s burden on a patient’s life [38].
Research suggests that regular contacts between physician and patient have a favorable implication on adherence [19]. An Italian prospective study investigated SLIT adherence in children and adolescents over 3 years in relation to the frequency of doctor’s visits. Patients visiting on a regular basis showed significantly lower withdrawal rates than those with fewer visits [39]. As a consequence, physician involvement and frequency of interaction between the patients and physician support better adherence [40]. With the understanding that adherence depends on settings and administration of selected AITs, it is best to be analyzed under uncontrolled real-life settings [40].
A recent real-world evidence (RWE) study generated adherence data for Germany, which we used to populate and to adapt an established cost effectiveness model [19, 21]. SCIT adherence in this RWE study in Germany was >60% at the end of the second and >35% at the completion of the third year for grasses. Adherence to SLIT was significantly lower both at 2 years (29.6–33.7%) and after 3 years (9.6–13.4%) for grass [19]. Based on these recent real-world data on adherence for preseasonal and seasonal AIT regimes, the model results suggest that a treatment of patients with pollen-induced rhinoconjunctivitis or AR using SCIT is both more effective and provides a better cost-effectiveness compared to a SLIT or a purely symptomatic treatment.
Patient relevant outcomes differences in QALYs could be identified as follows: patients receiving symptomatic treatment alone accumulated 7.89 QALYs over the modeling period, whereas SCIT and SLIT achieved 8.04 and 7.98 QALYs, respectively. These differences in QALYs are primarily due the differences in the percentage of patients completing the entire 3‑year SIT treatment period, and thereby profiting from the quality of life-enhancing and AA incidence-lowering effects of AIT [41].
Simulated incremental cost-effectiveness ratios (ICER) are in line with recently published data. However, compared to Di Bona et al. reporting an ICER of 11,418 € for SCIT and 15,212 € for SLIT, our results based on recent and representative RWE adherence data revealed a more superior cost-effectiveness of SCIT [42]. This might be due to differing assumptions compared to Di Bona et al.: first, we are not considering indirect costs, for example, valuing patient time on a monetary basis when visiting the doctor for injections as these are not considered by health insurance companies; second, different from our model Di Bona et al. assumed no diminishing of AIT efficacy when withdrawal from treatment occurred and third, we applied a half-cycle correction, i.e., those discontinuing in the first year were assumed to be valued at 50% only instead of the 100% in Di Bona’s analysis.
Vogelberg et al. observed a higher probability of developing asthma with SLIT compared to SCIT. However, we assumed a similar allergic asthma incidence in our modelling [19]. Hence, incorporating these differences would have been further advantageous for SCIT.
Several research teams have postulated that a clinically relevant effect of AIT is dependent on treatment duration achieved. If full treatment success is expected with at least three continuous seasonal cycles only [23, 36], early patient discontinuation considerably impacts the midterm cost-effectiveness of AIT, as the investment in year one or even year two without reaching year three could be considered a lost opportunity and nonrecoverable cost. However, to our knowledge no reliable clinical effectiveness data on a long-term annual basis are yet available to simulate this impact in an economic model.
Our findings are based on established health economic model calculations previously published by Reinhold et al. [7], who based the modeling framework on earlier versions of Verheggen et al. [15] and Westerhout et al. [22]. Comparable to Reinhold et al. we applied a product-based comparison for Germany instead of a SCIT treatment mix, which appears a more transparent comparison given the heterogeneity of individual SCIT preparations available on the market. Based on most recent data from country-specific pollen season information databases we could also adopt the length of the pollen season of Reinhold et al. of 4.5 months [21, 28].
Our analysis might be subject to limitations. Cost of asthma had to be annualized in this model based on AR and AA data from a 2003 study by Schramm et al., as more recent cost of disease data were not available [29]. This study concluded that the annual direct cost of treatment for adults with AR and SAR totals to 861 € resulting in an annualized current value of 1162 €, of which 292 € were attributable to AA.
We assumed 7 injections for administering SCIT. In the clinical trial by Corrigan et al. seven plus two maintenance injections were applied. However, even when using nine injections, the ICER of SCIT compared to ST changed from 7118 € to 7470 € only [31].
Furthermore, our model does not consider indirect costs, e.g., attributed to productivity loss, as this is not considered relevant from a third-party payor’s perspective in Germany.
Our model covers adult patients only. In general nonadherence is more common among adolescents [43]. Based on recent RWE adherence to SCIT is the highest in children, followed by adolescents, whereas adolescent SLIT patients show a lower adherence [19] Hence, the observed higher efficiency for SCIT in adult patients might be even more pronounced for nonadult patients.
Finally, due to scarcity of more granular clinical efficacy data our model does not account for partial annual treatment effects for those patients not being adherent over the full AIT course of 3 years.
Conclusion
Based on recent real-world evidence adherence data a SCIT allergoid preparation and a SLIT tablet were shown to be cost-effective in the treatment of patients with AR compared to symptomatic treatment alone in Germany. In our analysis, the SCIT treatment exhibits a better incremental cost-effectiveness than the SLIT tablet when compared to symptomatic treatment. A main reason for this is the difference in adherence to treatment in real life.
Abbreviations
- AA:
-
Allergic asthma
- AIT:
-
Allergen-specific immunotherapy
- AR:
-
Allergic rhinitis
- CEA:
-
Cost effectiveness analyses
- EBM:
-
Einheitlicher Bewertungsmaßstab (German remuneration scheme for statutory health insurance for accredited physicians)
- INCS:
-
Intranasal corticosteroids
- OAH:
-
Oral antihistamines
- QALY:
-
Quality-adjusted life year
- SCIT:
-
Subcutaneous immunotherapy
- SLIT:
-
Sublingual immunotherapy
- ST:
-
Symptomatic therapy
References
Ozdoganoglu T, Songu M, Inancli HM. Quality of life in allergic rhinitis. Ther Adv Respir Dis. 2012;6(1):25–39. https://doi.org/10.1177/1753465811424425.
Navarro A, Valero A, Julia B, Quirce S. Coexistence of asthma and allergic rhinitis in adult patients attending allergy clinics: ONEAIR study. J Investig Allergol Clin Immunol. 2008;18(4):233–8.
Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–98. https://doi.org/10.1111/all.13317.
Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72(11):1597–631. https://doi.org/10.1111/all.13201.
Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. https://doi.org/10.1186/1939-4551-7-6.
Halken S, Larenas-Linnemann D, Roberts G, Calderon MA, Angier E, Pfaar O, et al. EAACI guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28(8):728–45. https://doi.org/10.1111/pai.12807.
Arasi S, Corsello G, Villani A, Pajno GB. The future outlook on allergen immunotherapy in children: 2018 and beyond. Ital J Pediatr. 2018;44(1):80. https://doi.org/10.1186/s13052-018-0519-4.
Noon L. Prophylactic inoculation against hay fever. Int Arch Allergy Appl Immunol. 1953;4(4):285–8. https://doi.org/10.1159/000228032.
Passalacqua G, Bagnasco D, Canonica GW. 30 years of sublingual immunotherapy. Allergy. 2020;75(5):1107–20. https://doi.org/10.1111/all.14113.
Manzotti G, Riario-Sforza GG, Dimatteo M, Scolari C, Makri E, Incorvaia C. Comparing the compliance to a short schedule of subcutaneous immunotherapy and to sublingual immunotherapy during three years of treatment. Eur Ann Allergy Clin Immunol. 2016;48(6):224–7.
Linneberg A, Dam Petersen K, Hahn-Pedersen J, Hammerby E, Serup-Hansen N, Boxall N. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016;14:12. https://doi.org/10.1186/s12948-016-0049-9.
Pitt AD, Smith AF, Lindsell L, Voon LW, Rose PW, Bron AJ. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol. 2004;11(1):17–33. https://doi.org/10.1076/opep.11.1.17.26437.
Ronborg SM, Svendsen UG, Micheelsen JS, Ytte L, Andreasen JN, Ehlers L. Budget impact analysis of two immunotherapy products for treatment of grass pollen-induced allergic rhinoconjunctivitis. Clinicoecon Outcomes Res. 2012;4:253–60. https://doi.org/10.2147/CEOR.S34832.
Brüggenjürgen B, Reinhold T. Cost-effectiveness of grass pollen subcutaneous immunotherapy (SCIT) compared to sublingual immunotherapy (SLIT) and symptomatic treatment in Austria, Spain, and Switzerland. J Med Econ. 2018;21(4):374–81. https://doi.org/10.1080/13696998.2017.1419959.
Verheggen BG, Westerhout KY, Schreder CH, Augustin M. Health economic comparison of SLIT allergen and SCIT allergoid immunotherapy in patients with seasonal grass-allergic rhinoconjunctivitis in Germany. Clin Transl Allergy. 2015;5:1. https://doi.org/10.1186/s13601-015-0045-z.
Senna G, Caminati M, Lockey RF. Allergen immunotherapy adherence in the real world: How bad is it and how can it be improved? Curr Treat Options Allergy. 2015;2:14.
Incorvaia C, Mauro M, Leo G, Ridolo E. Adherence to sublingual immunotherapy. Curr Allergy Asthma Rep. 2016;16(2):12. https://doi.org/10.1007/s11882-015-0586-1.
Senna G, Ridolo E, Calderon M, Lombardi C, Canonica GW, Passalacqua G. Evidence of adherence to allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9(6):544–8. https://doi.org/10.1097/ACI.0b013e328332b8df.
Vogelberg C, Brüggenjürgen B, Richter H, Jutel M. Real-world adherence and evidence of subcutaneous and sublingual immunotherapy in grass and tree pollen-induced allergic rhinitis and asthma. Patient Prefer Adherence. 2020;14:817–27. https://doi.org/10.2147/PPA.S242957.
Cox LS, Murphey A, Hankin C. The cost-effectiveness of allergen immunotherapy compared with pharmacotherapy for treatment of allergic rhinitis and asthma. Immunol Allergy Clin North Am. 2020;40(1):69–85. https://doi.org/10.1016/j.iac.2019.09.003.
Reinhold T, Brüggenjürgen B. Cost-effectiveness of grass pollen SCIT compared with SLIT and symptomatic treatment. Allergo J Int. 2017;26(1):7–15. https://doi.org/10.1007/s40629-016-0002-y.
Westerhout KY, Verheggen BG, Schreder CH, Augustin M. Cost effectiveness analysis of immunotherapy in patients with grass pollen allergic rhinoconjunctivitis in Germany. J Med Econ. 2012;15(5):906–17. https://doi.org/10.3111/13696998.2012.688904.
Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long-term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. 2018;5(3):275–90. https://doi.org/10.1007/s40521-018-0176-2.
Werchan M, Werchan B, Bergmann K‑C. Deutscher Pollenflugkalender 4.0: Update der regionalen Pollenflugkalender 4.0 mit Messdaten von 2011 bis 2016. Allergo J. 2019;28(5):16–7. https://doi.org/10.1007/s15007-019-1887-9.
Allergopharma. FACHINFORMATION – Zusammenfassung der Merkmale des Arzneimittels (SPC) ALLERGOVIT® Gräser- und Getreidepollenpräparate. 2019. www.allergopharma.de. Accessed 6 Feb 2021.
Stallergenes. Product monograph—full prescribing information. 2017. http://www.oralair.com/hcp/materials/ORALAIR_Product_Monograph.pdf. Accessed 21 July 2017.
Shaaban R, Zureik M, Soussan D, Neukirch C, Heinrich J, Sunyer J, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372(9643):1049–57. https://doi.org/10.1016/S0140-6736(08)61446-4.
Stiftung Deutscher Polleninformationsdienst. Pollenflugkalender für Deutschland 2011–2016. 2018. http://www.pollenstiftung.de/pollenvorhersage/pollenflug-kalender/. Accessed 6 Feb 2021.
Schramm B, Ehlken B, Smala A, Quednau K, Berger K, Nowak D. Cost of illness of atopic asthma and seasonal allergic rhinitis in Germany: 1‑yr retrospective study. Eur Respir J. 2003;21(1):116–22. https://doi.org/10.1183/09031936.03.00019502.
IQWiG. Allgemeine Methoden Version 5.0. Gesundheitswesen. Köln: IQWiG; 2017.
Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A, Study G. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005;60(6):801–7. https://doi.org/10.1111/j.1398-9995.2005.00790.x.
Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol 2007;120:1338–45.
Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, Le Gall M. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol 2009;123:160–66.e3.
Didier A, Worm M, Horak F, Sussman G, de Beaumont O, Le Gall M, Melac M, Malling HJ. Sustained 3-year efficacy of pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol 2011;128:559–66.
Cox L, Wallace D. Specific allergy immunotherapy for allergic rhinitis: subcutaneous and sublingual. Immunol Allergy Clin North Am 2011;31:561–99.
Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41(9):1177–200. https://doi.org/10.1111/j.1365-2222.2011.03794.x.
Kiel MA, Gerth van Wijk R, Röder E, Al MJ, Hop WC, Rutten-van Mölken MP. Assessing the compliance and persistence of allergen immunotherapy in allergic rhinitis using a retrospective pharmacy database from the Netherlands. Value Health. 2011;14(7):A233.
Baiardini I, Novakova S, Mihaicuta S, Oguzulgen IK, Canonica GW. Adherence to treatment in allergic respiratory diseases. Expert Rev Respir Med. 2018; https://doi.org/10.1080/17476348.2019.1554438.
Vita D, Caminiti L, Ruggeri P, Pajno GB. Sublingual immunotherapy: adherence based on timing and monitoring control visits. Allergy. 2010;65(5):668–9. https://doi.org/10.1111/j.1398-9995.2009.02223.x.
Egert-Schmidt AM, Kolbe JM, Mussler S, Thum-Oltmer S. Patients’ compliance with different administration routes for allergen immunotherapy in Germany. Patient Prefer Adherence. 2014;8:1475–81. https://doi.org/10.2147/PPA.S70326.
Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30(1):48–53. https://doi.org/10.2500/ajra.2016.30.4253.
Di Bona D, Bilancia M, Albanesi M, Caiaffa MF, Macchia L. Cost-effectiveness of grass pollen allergen immunotherapy in adults. Allergy. 2020; https://doi.org/10.1111/all.14246.
Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190–9. https://doi.org/10.1016/j.pec.2017.05.037.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Brüggenjürgen has received honoraria for lectures, workshops, and commissioned research from Allergopharma GmbH & Co. KG, ALK-Abelló, and Stallergènes. L. Klimek reports grants and personal fees from Allergopharma, grants and personal fees from MEDA/Mylan, personal fees from HAL Allergie, personal fees from ALK Abelló, grants and personal fees from LETI Pharma, grants and personal fees from Stallergenes, grants from Quintiles, grants and personal fees from Sanofi, grants from ASIT biotech, grants from Lofarma, personal fees from Allergy Therapeut., grants from AstraZeneca, grants and personal fees from GSK, grants from Inmunotek, personal fees from Cassella med, personal fees from Novartis, outside the submitted work. T. Reinhold received honoraria for lectures from Allergopharma GmbH & Co. KG. This study was sponsored by Allergopharma GmbH & Co. KG.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brüggenjürgen, B., Klimek, L. & Reinhold, T. Real world effectiveness and cost consequences of grass pollen SCIT compared with SLIT and symptomatic treatment. Allergo J Int 30, 198–206 (2021). https://doi.org/10.1007/s40629-021-00183-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-021-00183-5