Abstract
Background
Dysthyroid optic neuropathy (DON) is a serious complication of Graves orbitopathy (GO) from optic nerve dysfunction that may result in permanent loss of vision.
Purpose
This paper reviews the current knowledge of DON, including its pathogenesis and epidemiology, clinical and radiologic features, and management choices and outcomes.
Methods
Literature review and author retrospective case series.
Results
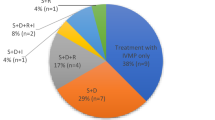
Over 90% of DON cases are related to nerve compression by enlarged extraocular muscles (EOM) while the remainder are caused by stretching of the optic nerve without compression. DON’s incidence is 5–8% of GO cases. Risk factors include advancing age, male gender, smoking and diabetes mellitus, and these cases should be referred promptly to an ophthalmologist or GO clinic to rule out DON and for ongoing care. Clinical features of DON may include reduction in central and colour vision (unexplained by other ocular disorders), afferent pupil defect and/or optic disc edema. Since most cases are associated with enlarged EOM, restricted motility and soft tissue venous congestion are common. Visual fields and optical coherence tomography (OCT) help confirm the diagnosis while CT or MRI Scans show apical optic nerve compression or proptosis with optic nerve stretch. Standard therapy includes iv/oral corticosteroids (CS) with partial response in most cases, but often relapse with tapering. Radiotherapy may delay or avoid surgery and may prevent the onset of DON when combined with CS in high-risk individuals. The benefits of newer biologic targeted therapy are not clear. Orbital decompression surgery often has positive outcomes, even in cases of severe vision loss or delayed surgery. The most common surgical complication is worsening strabismus, which may worsen visual function and quality of life. In rare cases, permanent vision loss from DON may occur despite full therapy.
Conclusions
Although DON may cause vision loss, most cases are reversible if recognized and managed in a timely manner.







Similar content being viewed by others
References
McKeag D, Lane CM, Lazarus JH et al (2007) Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy survey. Br J Ophthalmol 91:455–458
Dolman PJ (2012) Evaluating graves orbitopathy. Best Pract Res Clin Endocrinol Metab 26(3):229–248
Saeed P, Tavakoli Rad S, Bisschop PHLT (2018) Dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 34(4 Suppl 1):S60–S67
Blandford AD, Zhang D, Chundury RV, Perry JD (2017) Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 12(2):111–121
Bahn RS (2015) Current insights into the pathogenesis of Graves’ ophthalmopathy. Horm Metab Res 47(10):773–778
Smith TJ (2020) Teprotumumab in thyroid-associated ophthalmopathy: rationale for therapeutic insulin-like growth factor-I receptor inhibition. J Neuroophthalmol 40(1):74–78
Dolman PJ (2018) Grading severity and activity in thyroid eye disease. Ophthal Plast Reconstr Surg 34(4S):S34–S40
Davies MJ, Dolman PJ (2016) Levator muscle enlargement in thyroid eye disease-related upper eyelid retraction. Ophthalmic Plast Reconstr Surg 33(1):35–39
Dolman PJ, Rootman J (2005) Predictors of disease severity in thyroid-related orbitopathy. (chap18) Orbital Disease. Present status and future challenges. Taylor and Francis
Rundle FF (1960) Ocular changes in Graves’ disease. QJM 29:113–126
Bartalena L et al (2008) Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 158:273–285
Mourits MP, Prummel MF, Wiersinga WM et al (1997) Clinical activity score as a guide in the management of patients with Graves’ Ophthalmopathy. Clin Endocrinol 47:9
Dolman PJ, Rootman J (2006) VISA classification for Graves orbitopathy. Ophthalmic Plast Reconstr Surg 22(5):319–324
Day RM, Carroll FD (1967) Corticosteroids in the treatment of optic nerve involvement associated with thyroid dysfunction. Trans Am Ophth Soc 65:41–51
Kennerdell JS, Rosenbaum AE, El-Hoshy MH (1981) Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch Ophthalmol 99(5):807–809
Kazim M, Trokel SL, Acaroglu G, Elliott A (2000) Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol 84(6):600–605
Rose GE, Vahdani K (2020) Optic nerve stretch is unlikely to be a significant causative factor in dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 36(2):157–163
Wong Y, Dickinson J, Perros P et al (2018) A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom. Eye 32(10):1555–1562
Dolman PJ (2019) Dysthyroid optic neuropathy: evaluation and management. Hong Leong Professorship Special Lecture. IGOS 4th International Symposium, Singapore
Khong JJ, Finch S, De Silva C et al (2016) Risk factors for Graves’ orbitopathy; the Australian Thyroid-Associated Orbitopathy Research (ATOR) Study. J Clin Endocrinol Metab 1010(7):2711–2720
Kalmann R, Mourits MP (1999) Diabetes mellitus: a risk factor in patients with Graves' orbitopathy. Br J Ophthalmol 83(4):463–465
Shams PN, Ma R, Pickles T, Rootman J (2014) Dolman PJ Reduced risk of compressive optic neuropathy using orbital radiotherapy in patients with active thyroid eye disease. Am J Ophthalmol 157(6):1299–1305
Dickinson AJ, Hintschich C (2017) In: Wiersinga WM, Kahaly GJ (eds) Graves’ orbitopathy: a multidisciplinary approach—questions and answers. Karger, Basel, pp 19–20
Weis E, Heran MK, Jhamb A, Chan AK, Chiu JP, Hurley MC, Rootman J (2011) Clinical and soft-tissue computed tomographic predictors of dysthyroid optic neuropathy: refinement of the constellation of findings at presentation. Arch Ophthalmol 29(10):1332–1336
Choi CJ, Oropesa S, Callahan AB, Glass LR et al (2017) Patterns of visual field changes in thyroid eye disease. Orbit 36(4):201–207
Lao TW, Rong SS, Ling AN, Brelén ME et al (2019) Electrophysiological studies in thyroid associated orbitopathy: a systematic review. Curr Opin Neurol 32(1):115–123
Micieli JA, Newman NJ, Biousse V (2017) The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Expert Rev Ophthalmol 12(2):111–121
Giaconi JA, Kazim M, Rho T et al (2002) CT scan evidence of dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 18(3):177–182
Oropesa S, Dunbar KE, Godfrey KJ, Callahan AB, Campbell AA, Kazim M (2018) Predominant contribution of superior rectus-levator complex enlargement to optic neuropathy and inferior visual field defects in thyroid eye disease. Ophthalmic Plast Reconstr Surg 35:262–265
Callahan AB, Campbell AA, Oropesa S et al (2018) The Columbia thyroid eye disease-compressive optic neuropathy diagnostic formula. Ophthalmic Plast Reconstr Surg 34(4 Suppl 1):S68–S71
Zhao LQ, Yu DY, Cheng JW (2019) Intravenous glucocorticoids therapy in the treatment of Graves’ ophthalmopathy: a systematic review and meta-analysis. Int J Ophthalmol 12(7):1177–1186
Bartalena L, Krassas GE, Wiersinga W, Marcocci C et al (2012) (2012) European Group on Graves’ Orbitopathy. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 97(12):4454–4463
Dolman PJ, Rath S (2012) Orbital radiotherapy for thyroid eye disease. Curr Opin Ophthalmol 23(5):427–432
Gold KG, Scofield S, Isaacson SR, Stewart MW, Kazim M (2018) Orbital radiotherapy combined with corticosteroid treatment for thyroid eye disease-compressive optic neuropathy. Ophthalmic Plast Reconstr Surg 34(2):172–177
Salvi M, Vannucchi G, Curro N et al (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate-severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 100:402–431
Stan MN, Garrity JA, Carranza Leon BG, Prabin T et al (2015) Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 100(2):432–441
Bartalena L, Baldeschi L, Boboridis K, Eckstein A et al (2016) The 2016 European Thyroid Association/European Group on Graves’ orbitopathy guidelines for the management of graves' orbitopathy. Eur Thyroid J 5:9–26
Khanna D, Chong KK, Afifiyan NF, Hwang CJ et al (2010) Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology 117(1):133–139
Perez-Moreiras JV et al (2018) Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol 195:181–190
Douglas RS, Kahaly GJ, Patel A, Sile S et al (2020) Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 382:341–352
Tooley AA, Godfrey KJ, Kazim M (2019) Evolution of thyroid eye disease decompression-dysthyroid optic neuropathy. Eye (Lond) 33(2):206–211
Jefferis JM, Jones RK, Currie ZI, Tan JH, Salvi SM (2018) Orbital decompression for thyroid eye disease: methods, outcomes, and complications. Eye 32:626–636
Choe CH, Cho R, Elner VM (2011) Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthalmic Plast Reconstr Surg 27(1):4–11
Boboridis KG, Bunce C (2011) Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev. 12:CD007630
Chu EA, Miller NR, Lane AP (2009) Selective endoscopic decompression of the orbital apex for dysthyroid optic neuropathy. Laryngoscope 119(6):1236–1240
Liao SL, Chang TC, Lin LL (2006) Transcaruncular orbital decompression: an alternate procedure for Graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol 141(5):810–818
Jeon C, Shin JH, Woo KI, Kim YD (2012) Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean J Ophthalmol 26(2):73–79
Badilla J, Dolman PJ (2008) Intracranial hemorrhage complicating an orbital decompression. Orbit 27(2):143–145
Badilla J, Dolman PJ (2007) Cerebrospinal fluid leaks complicating orbital or oculoplastic surgery. Arch Ophthalmol 125(12):1631–1634
Fayers T, Dolman PJ (2011) Validity and reliability of the TED-QOL: a new three-item questionnaire to assess quality of life in thyroid eye disease. Br J Ophthalmol 95(12):1670–1674
Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM (1998) Development of a disease specific quality of life questionnaire for patients with Graves’ ophthalmopathy: the GO-QOL. Br J Ophthalmol 82(7):773–779
Fayers T, Fayers PM, Dolman PJ (2016) Sensitivity and responsiveness of the patient-reported TED-QOL to rehabilitative surgery in thyroid eye disease. Orbit 35(6):328–333
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author confirms there are no conflicts of interest.
Ethics approval
Not required for review article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dolman, P.J. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest 44, 421–429 (2021). https://doi.org/10.1007/s40618-020-01361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01361-y