Abstract
Purpose
Gitelman syndrome (GS) is an autosomal recessive renal tubular disease that arises as a consequence of mutations in the SLC12A3 gene, which codes for an Na–Cl cotransporter (NCC) in distal renal tubules. This study was designed to explore the mutations associated with GS in an effort to more fully understand the molecular mechanisms governing GS.
Methods
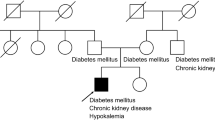
We analyzed SLC12A3 mutations in a pedigree including a 42-year-old male with GS as well as four related family members over three generations using Sanger and next generation sequencing approaches. We additionally explored the functional ramifications of identified mutations using both Xenopus oocytes and the HEK293T cell line.
Results
We found that the subject with GS exhibited characteristic symptoms including sporadic thirst, fatigue, excess urination, and substantial hypokalemia and hypocalciuria, although magnesium levels were normal. Other analyzed subjects in this pedigree had normal laboratory findings and did not exhibit clear signs of GS. Sequencing analyses revealed that the GS subject exhibited a homozygous missense mutation (c.2874C > G, p.N958K) in exon 24 of SLC12A3. Both parents of this GS subject, as well as his older brother and daughter all exhibited heterozygous mutations at this same site. Functional analyses in Xenopus oocytes indicated that this mutated SLC12A3 gene encodes a protein which fails to mediate normal sodium transport, and when this mutant gene was expressed in HEK293T cells, we observed significant increases in endoplasmic reticulum (ER)-stress pathway activation.
Conclusion
The p.N958K mutation in exon 24 of SLC12A3 can trigger GS at least in part via enhancing ER stress responses.




Similar content being viewed by others
References
Knoers NV, Levtchenko EN (2008) Gitelman syndrome. Orphanet J Rare Dis 3:22
Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ et al (2007) Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 18:1271–1283
Cruz DN, Shaer AJ, Bia MJ, Lifton RP, Simon DB et al (2001) Gitelman's syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int 59:710–717
Chen Q, Wu Y, Zhao J, Jia Y, Wang W (2018) A case of hypokalemia and proteinuria with a new mutation in the SLC12A3 Gene. BMC Nephrol 19:275
Urbanova M, Reiterova J, Stekrova J, Lnenicka P, Rysava R (2011) DNA analysis of renal electrolyte transporter genes among patients suffering from Bartter and Gitelman syndromes: summary of mutation screening. Folia Biol 57:65–73
Sinha A, Lnenicka P, Basu B, Gulati A, Hari P et al (2012) Gitelman syndrome: novel mutation and long-term follow-up. Clin Exp Nephrol 16:306–309
Subasinghe CJ, Sirisena ND, Herath C, Berge KE, Leren TP et al (2017) Novel mutation in the SLC12A3 gene in a Sri Lankan family with Gitelman syndrome & coexistent diabetes: a case report. BMC Nephrol 18:140
Fanis P, Efstathiou E, Neocleous V, Phylactou LA, Hadjipanayis A (2019) A novel heterozygous duplication of the SLC12A3 gene in two Gitelman syndrome pedigrees: indicating a founder effect. J Genet 98:5
Wang CL (2019) Novel heterozygous missense mutation of SLC12A3 gene in Gitelman syndrome: a case report. World J Clin cases 7:1522–1528
Lu Q, Zhang Y, Song C, An Z, Wei S et al (2016) A novel SLC12A3 gene homozygous mutation of Gitelman syndrome in an Asian pedigree and literature review. J Endocrinol Invest 39:333–340
Blanchard A, Vargas-Poussou R, Vallet M, Caumont-Prim A, Allard J et al (2015) Indomethacin, amiloride, or eplerenone for treating hypokalemia in Gitelman syndrome. J Am Soc Nephrol 26:468–475
Wang F, Shi C, Cui Y, Li C, Tong A (2017) Mutation profile and treatment of Gitelman syndrome in Chinese patients. Clin Exp Nephrol 21:293–299
Valdez-Flores MA, Vargas-Poussou R, Verkaart S, Tutakhel OA, Valdez-Ortiz A et al (2016) Functionomics of NCC mutations in Gitelman syndrome using a novel mammalian cell-based activity assay. Am J Physiol Renal Physiol 311:F1159–F1167
Kunchaparty S, Palcso M, Berkman J, Velazquez H, Desir GV et al (1999) Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman's syndrome. Am J Physiol 277:F643–649
Paredes A, Plata C, Rivera M, Moreno E, Vazquez N et al (2006) Activity of the renal Na+-K+-2Cl- cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl- transport. Am J Physiol Renal Physiol 290:F1094–1102
Moremen KW, Tiemeyer M, Nairn AV (2012) Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol 13:448–462
Dvela-Levitt M, Kost-Alimova M, Emani M, Kohnert E, Thompson R et al (2019) Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell 178(521–535):e523
Siwecka N, Rozpedek W, Pytel D, Wawrzynkiewicz A, Dziki A et al (2019) Dual role of endoplasmic reticulum stress-mediated unfolded protein response signaling pathway in Carcinogenesis. Int J Mol Sci 20(18):4354
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology 22:193–201
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35:373–381
Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC et al (2008) Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell Microbiol 10:1775–1786
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Sriburi R, Jackowski S, Mori K, Brewer JW (2004) XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J cell Biol 167:35–41
Halperin D, Kadir R, Perez Y, Drabkin M, Yogev Y et al (2019) SEC31A mutation affects ER homeostasis, causing a neurological syndrome. J Med Genet 56:139–148
Kong Y, Xu K, Yuan K, Zhu J, Gu W et al (2019) Digenetic inheritance of SLC12A3 and CLCNKB genes in a Chinese girl with Gitelman syndrome. BMC Pediatr 19:114
Dimke H (2011) Exploring the intricate regulatory network controlling the thiazide-sensitive NaCl cotransporter (NCC). Pflugers Arch 462:767–777
Cipolletta E, Di Matteo A, Filippucci E, Grassi W (2019) Calcium Pyrophosphate Deposition Disease in a Patient with Familial Hypokalemia-Hypomagnesemia (Gitelman's-Syndrome): a Case Report. Ultraschall Med. https://doi.org/10.1055/a-0990-9960
Tosi F, Bianda ND, Truttmann AC, Crosazzo L, Bianchetti MG et al (2004) Normal plasma total magnesium in Gitelman syndrome. Am J Med 116:573–574
Jiang L, Chen C, Yuan T, Qin Y, Hu M et al (2014) Clinical severity of Gitelman syndrome determined by serum magnesium. Am J Nephrol 39:357–366
Schnoz C, Carrel M, Loffing J (2019) Loss of sodium chloride co-transporter impairs the outgrowth of the renal distal convoluted tubule during renal development. Nephrol Dial Transpl 35(3):411–432
Zeng Y, Li P, Fang S, Wu C, Zhang Y et al (2019) Genetic Analysis of SLC12A3 Gene in Chinese Patients with Gitelman Syndrome. Med Sci Monit 25:5942–5952
Fulchiero R, Seo-Mayer P (2019) Bartter Syndrome and Gitelman Syndrome. Pediatr Clin North Am 66:121–134
Bao M, Cai J, Yang X, Ma W (2019) Genetic screening for Bartter syndrome and Gitelman syndrome pathogenic genes among individuals with hypertension and hypokalemia. Clin Exp Hypertens 41:381–388
Pathare G, Anderegg M, Albano G, Lang F, Fuster DG (2018) Elevated FGF23 Levels in Mice Lacking the Thiazide-Sensitive NaCl cotransporter (NCC). Sci Rep 8:3590
Zhou H, Liang X, Qing Y, Meng B, Zhou J et al (2018) Complicated Gitelman syndrome and autoimmune thyroid disease: a case report with a new homozygous mutation in the SLC12A3 gene and literature review. BMC Endocr Disord 18:82
Wichmann L, Dulai JS, Marles-Wright J, Maxeiner S, Szczesniak PP et al (2019) An extracellular acidic cleft confers profound H(+)-sensitivity to epithelial sodium channels containing the delta-subunit in Xenopus laevis. J Biol Chem 294:12507–12520
Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP et al (1995) A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci USA 92:5699–5703
Yang CL, Zhu X, Ellison DH (2007) The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Investig 117:3403–3411
Needham PG, Mikoluk K, Dhakarwal P, Khadem S, Snyder AC et al (2011) The thiazide-sensitive NaCl cotransporter is targeted for chaperone-dependent endoplasmic reticulum-associated degradation. J Biol Chem 286:43611–43621
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Investig 115:2656–2664
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917
Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D et al (2019) Endoplasmic reticulum stress signaling—from basic mechanisms to clinical applications. FEBS J 286:241–278
Graham JB, Canniff NP, Hebert DN (2019) TPR-containing proteins control protein organization and homeostasis for the endoplasmic reticulum. Crit Rev Biochem Mol Biol 54:103–118
Funding
This study was supported by Grants from the young science foundation of national natural science foundation of China (81800865).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Changzheng hospital, and all pedigree members gave informed consent to participate.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, W., Huang, X., Liu, Y. et al. A novel homozygous mutation (p.N958K) of SLC12A3 in Gitelman syndrome is associated with endoplasmic reticulum stress. J Endocrinol Invest 44, 471–480 (2021). https://doi.org/10.1007/s40618-020-01329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01329-y