Abstract
Purpose
Glucagon-like peptide 1 (GLP-1) is an incretin hormone that appears to play a major role in the control of food intake. The aim of this investigation was to evaluate and quantify the association of circulating GLP-1 concentration with ad libitum total calorie and macronutrient intake.
Methods
One-hundred and fifteen individuals (72 men) aged 35 ± 10 years were admitted for an inpatient study investigating the determinants of energy intake. Ad libitum food intake was assessed during 3 days using a reproducible vending machine paradigm. Fasting plasma GLP-1 concentrations were measured on the morning of the first day and on the morning of the fourth day after ad libitum feeding.
Results
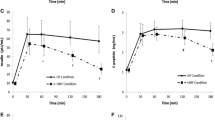
Plasma GLP-1 concentrations increased by 14% after 3 days of ad libitum food intake. Individuals overate on average 139 ± 45% of weight-maintaining energy needs. Fasting plasma GLP-1 on day 1 was negatively associated with carbohydrate intake (r = − 0.2, p = 0.03) and with daily energy intake from low fat–high simple sugar (r = − 0.22, p = 0.016).
Conclusion
Higher plasma GLP-1 concentrations prior to ad libitum food intake were associated with lower carbohydrate intake and lower simple sugar ingestion, indicating a possible role of the GLP-1 in the reward pathway regulating simple sugar intake.
Trial registration
ClinicalTrials.gov identifier: NCT00342732.




Similar content being viewed by others
Abbreviations
- GLP-1:
-
Glucagon-like peptide 1
- FM:
-
Fat mass
- FFM:
-
Fat-free mass
- OGTT:
-
Oral glucose tolerance test
- DXA:
-
Dual energy X-ray absorptiometry
- CNS:
-
Central nervous system
- LF:
-
Low fat
- HSS:
-
High simple sugar
- HF:
-
High fat
- HCC:
-
High complex carbohydrate
- HP:
-
Low fat/high protein
References
Zheng W, McLerran DF, Rolland B et al (2011) Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 364:719–729
Ravussin E, Bogardus C (2000) Energy balance and weight regulation: genetics versus environment. Br J Nutr 83:S17–S20
Poggiogalle E, Donini L, Chiesa C et al (2018) Does endogenous GLP-1 affect resting energy expenditure and fuel selection in overweight and obese adults? J Endocrinol Investig 41:439–445
Abbott CR, Monteiro M, Small CJ et al (2005) The inhibitory effects of peripheral administration of peptide YY 3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal–brainstem–hypothalamic pathway. Brain Res 1044:127–131
Flint A, Raben A, Astrup A, Holst JJ (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Investig 101:515
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439
Kreymann B, Ghatei M, Williams G, Bloom S (1987) Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 330:1300–1304
De Heer J, Rasmussen C, Coy D, Holst JJ (2008) Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51:2263
Alssema M, Rijkelijkhuizen JM, Holst JJ et al (2013) Preserved GLP-1 and exaggerated GIP secretion in type 2 diabetes and relationships with triglycerides and ALT. Eur J Endocrinol 169:421–430
Verdich C, Flint A, Gutzwiller J-P et al (2001) A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 86:4382–4389
Zander M, Madsbad S, Madsen JL, Holst JJ (2002) Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830
Parry SA, Smith JR, Corbett TR, Woods RM, Hulston CJ (2017) Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. Br J Nutr 117:48–55
Brøns C, Jensen CB, Storgaard H et al (2009) Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587:2387–2397
Wadden D, Cahill F, Amini P et al (2013) Circulating glucagon-like peptide-1 increases in response to short-term overfeeding in men. Nutr Metab 10:33
Piaggi P, Thearle MS, Krakoff J, Votruba SB (2015) Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab 100:3011–3020
Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E (1991) Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 53:1368–1371
Gavin JR III, Alberti K, Davidson MB, DeFronzo RA (1997) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20:1183
Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD (2010) Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr 91:343–348
Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR (1998) Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav 63:919–928
Basolo A, Burkholder J, Osgood K et al (2018) Exenatide has a pronounced effect on energy intake but not energy expenditure in non-diabetic subjects with obesity: a randomized, double-blind, placebo-controlled trial. Metabolism 85:116–125. https://doi.org/10.1016/j.metabol.2018.03.017
Astrup A, Carraro R, Finer N et al (2005) Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analogue, liraglutide. Int J Obes 2012(36):890
Näslund E, Barkeling B, King N et al (1999) Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord 23(3):304–311
Zanchi D, Depoorter A, Egloff L et al (2017) The impact of gut hormones on the neural circuit of appetite and satiety: a systematic review. Neurosci Biobehav Rev 80:457–475
Wang G-J, Volkow ND, Thanos PK, Fowler JS (2009) Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med 3:8
Pritchett CE, Hajnal A (2012) Glucagon-like peptide-1 regulation of carbohydrate intake is differentially affected by obesogenic diets. Obesity 20:313–317
Sandoval DA, Bagnol D, Woods SC, D’alessio DA, Seeley RJ (2008) Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57:2046–2054
Hayes MR, Bradley L, Grill HJ (2009) Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150:2654–2659
Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280
Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, Skibicka KP (2015) Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One 10:e0119034
Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012) The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32:4812–4820
Hayes MR, Kanoski SE, De Jonghe BC et al (2011) The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol 301:R1479–R1485
Shin YK, Martin B, Golden E et al (2008) Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106:455–463
Bachmanov AA, Beauchamp GK (2007) Taste receptor genes. Annu Rev Nutr 27:389–414
Young RL (2011) Sensing via intestinal sweet taste pathways. Front Neurosci 5:23. https://doi.org/10.3389/fnins.2011.00023
Sclafani A (2007) Sweet taste signaling in the gut. Proc Natl Acad Sci 104:14887–14888
Zheng H, Berthoud H-R (2007) Eating for pleasure or calories. Curr Opin Pharmacol 7:607–612
Berthoud H-R, Morrison C (2008) The brain, appetite, and obesity. Annu Rev Psychol 59:55–92
Brands M, Swat M, Lammers NM et al (2013) Effects of a hypercaloric diet on β-cell responsivity in lean healthy men. Clin Endocrinol 78:217–225
He J, Votruba S, Pomeroy J, Bonfiglio S, Krakoff J (2012) Measurement of ad libitum food intake, physical activity, and sedentary time in response to overfeeding. PLoS One 7:e36225
Velasquez-Mieyer P, Cowan P, Umpierrez G, Lustig R, Cashion A, Burghen G (2003) Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. Int J Obes Relat Metab Disord J Int Assoc Study Obes 27:1359
Yabe D, Kuroe A, Lee S et al (2010) Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Investig 1:56–59
Sleddering MA, Bakker LE, Janssen LG, Meinders AE, Jazet IM (2014) Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism 63:226–232
Heijboer AC, Frans A, Lomecky M, Blankenstein MA (2011) Analysis of glucagon-like peptide 1; what to measure? Clin Chim Acta 412:1191–1194
Acknowledgements
The authors thank the volunteers who participated in our studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations. Also, the authors thank the metabolic kitchen stuff. This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors have nothing to disclose.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Contributions
AB analyzed and interpreted data and wrote the manuscript. PP and ES assisted with the interpretation of the data and revised the manuscript. MH and SH supported with the interpretation of the data and reviewed the manuscript. SV and JK designed, implemented and conducted the study. All authors read and approved the final manuscript. All authors critically revised the draft and approved the final manuscript. AB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Basolo, A., Heinitz, S., Stinson, E.J. et al. Fasting glucagon-like peptide 1 concentration is associated with lower carbohydrate intake and increases with overeating. J Endocrinol Invest 42, 557–566 (2019). https://doi.org/10.1007/s40618-018-0954-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0954-5