Abstract
Objectives
To compare the diagnostic value of the SARC-F, MRSA-7 and MRSA-5 questionnaires in screening for sarcopenia in inpatients with chronic heart failure (CHF).
Patients
A total of 355 CHF patients hospitalized from January 2019 to August 2019 who met the study’s selection criteria were included in the analysis.
Measurements
Handgrip strength and gait speed were measured, and bioelectrical impedance analysis (BIA) was used to estimate appendicular skeletal muscle mass. The sensitivity/specificity of the SARC-F, MRSA-7 and MRSA-5 questionnaires was evaluated.
Results
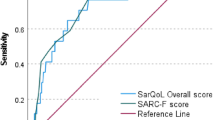
The diagnostic criteria of the Asia Working Group for Sarcopenia (AWGS) were used as the gold standard for diagnosing sarcopenia. The prevalence of sarcopenia was 55.8% according to the AWGS diagnostic criteria, 31.0% according to the SARC-F, 73.0% according to the MRSA-7, and 71.3% according to the MRSA-5. Using the AWGS criteria as the gold standard, the SARC-F had a sensitivity of 52.5% and a specificity of 96.2% in the whole study population, the MRSA-7 had a sensitivity of 92.4% and a specificity of 51.6%, and the MRSA-5 had a sensitivity of 93.9% and a specificity of 57.3%. The areas under the ROC curves for the SARC-F, MRSA-7 and MRSA-5 were 0.78, 0.74 and 0.77, respectively.
Conclusions
The MSRA-7 and MSRA-5 may serve as novel screening tools for sarcopenia in hospitalized patients with CHF. The SARC-F, a classic screening tool, is also suitable for this population. The MSRA-7 and MSRA-5 have better sensitivity, whereas the SARC-F has better specificity.
Similar content being viewed by others
Introduction
Heart failure (HF) is a common chronic disease that is the terminal stage of all kinds of heart disease, and the incidence and prevalence of HF are increasing year by year. In recent years, the peripheral consequences of HF have received more attention [1]. When HF occurs, myocardial contractility decreases, peripheral skeletal muscle blood perfusion is insufficient, and skeletal muscle mass and muscle function decline, leading to sarcopenia. Sarcopenia is a syndrome associated with ageing characterized by a progressive and overall loss of skeletal muscle and strength, which may lead to disability, reduced quality of life and even death. Patients with HF combined with sarcopenia often have weakened muscle strength and decreased exercise ability and are prone to develop weakness and cardiogenic cachexia, increasing the rate of rehospitalization and mortality [2, 3]. Research on sarcopenia in China started late, and research on chronic heart failure (CHF) combined with sarcopenia is limited. Early screening and active intervention are necessary to improve the prognosis of patients with HF complicated with sarcopenia.
According to the gold standard for diagnosing sarcopenia, the diagnosis of sarcopenia relies on large pieces of medical equipment, which is expensive and uncommon. Therefore, testing for sarcopenia cannot be widely promoted, and a simple method for performing a preliminary screening for sarcopenia is needed in clinical practice. Simple methods for screening for sarcopenia include body function assessments, muscle function assessment scales and tools, and questionnaire surveys. Because of the physical limitations of CHF patients, many patients cannot complete body function assessments. The scoring criteria for muscle function assessment tools in the scale category are complex, and there are many scoring principles that are susceptible to the influence of the tester’s motivation. The SARC-F (Supplementary Table S1), MRSA-7 and MRSA-5 (Supplementary Table S2) questionnaires are easy to understand and are not restricted by the health status of the patients, so they are suitable for wide application. However, the accuracy of these three questionnaires has only been verified in healthy people living in the community, and few studies have been conducted in patients with CHF. The purpose of this study was to clarify the accuracy of the three sarcopenia questionnaires in screening for sarcopenia in hospitalized patients with CHF.
Methods
Study design and population
A total of 355 consecutive CHF patients hospitalized at the Second Affiliated Hospital of Nanjing Medical University from January 2019 to August 2019, including 187 maintenance haemodialysis (HD) patients, were included. The inclusion criteria were as follows: (1) meeting the 2018 Chinese guidelines for the diagnosis and treatment of HF [4]; presence of typical HF symptoms, exhibiting typical signs of HF, having left ventricular ejection fraction (LVEF) < 40% or LVEF normal or only slightly reduced but associated with structural heart disease and/or diastolic cardiac dysfunction, or having NT-proBNP > 400 pg/ml or BNP > 150 pg/ml; and (2) age ≥ 60 years old. The exclusion criteria were as follows: (1) inability to communicate with researchers or obtain informed consent; (2) major diseases, such as tumours, severe weakness, and survival expectancy of less than 6 months; (3) severe infectious diseases and systemic immune diseases (including systemic lupus erythematosus, connective tissue disease, multiple myeloma, amyloidosis, rheumatism, rheumatoid disease, etc.); (4) acute coronary syndrome and acute HF; (5) chronic pulmonary diseases and liver insufficiency; (6) pregnancy; (7) skin damage at the contact place of the BIA electrode; (8) motor impairment and cognitive impairment; and (9) HF combined with neuromuscular diseases that affect muscle strength measurement or require the long-term use of drugs that affect body weight, such as glucocorticoids.
Measurement of handgrip strength, gait speed, and skeletal muscle mass
After the patient’s condition was stable and there was no oedema throughout the body, the following measurements were conducted by the researchers before discharge. For the handgrip strength measurement, the patient was seated, and the feet were placed naturally on the ground. The hip was bent 90°, with the upper arm and chest flat, the forearm in a neutral position, the wrist extended 0°–30°, and the feet maintaining a 0°–15° deviation. A mechanical grip dynamometer (xiangshan) was used to measure the strength of the dominant hand twice, and the larger value was recorded. For the gait speed measurement, patients were instructed to walk at a natural speed for 4 m indoors, and the time spent was recorded. The measurement was repeated twice, and the faster value was recorded. The appendicular skeletal muscle mass (ASM) was measured using a bioimpedance analysis (BIA) device (InBody S10, Biospace, Korea). Repeated BIA measurements were performed on all patients. Then, the average of the two measurements was used as the ASM value. The skeletal muscle mass index (SMI) was then obtained by dividing the ASM by height squared. To ensure the same general conditions (clothing, fasting, etc.) during the measurement, two measurements of the same patient were completed within 30 min, and both measurements were conducted by the same researcher. All these tests were performed by a trained researcher.
Assessment of sarcopenia using different criteria
The diagnostic criteria of AWGS were used as the gold standard for diagnosing sarcopenia (Supplementary Table S3). Next, the SARC-F, MRSA-7, and MRSA-5 were used to estimate sarcopenia. A total score of SARC-F ≥ 4 indicated sarcopenia [5]. A total score of MRSA-7 ≤ 30 and a total score of MRSA-5 ≤ 45 indicated sacopenia [6].
Covariates
Trained nurses collected the following covariates via face-to-face interviews: age, sex, and medical history of the following chronic diseases: hypertension, diabetes, coronary heart disease and stroke. Additionally, body weight and height were measured using a stadiometer and a digital floor scale to the nearest 0.1 kg and 0.1 cm, respectively.
Statistical analyses
All statistical analyses were performed with SPSS 20.0 (IBM Corp, Armonk, NY). All statistical tests were two-sided. A p value of < 0.001 indicated significance. We also used the ROC curve to compare the overall diagnostic accuracy of the SARC-F, MRSA-7, and MRSA-5. We calculated the area under the ROC curve (AUC).
Results
Prevalence of sarcopenia
The prevalence of sarcopenia was 55.8% according to the AWGS diagnostic criteria, 31.0% according to the SARC-F, 73.0% according to the MRSA-7, and 71.3% according to the MRSA-5.
Patient clinical data
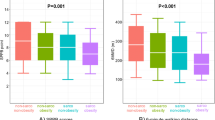
Characteristics of the patients are presented in Table 1. The mean scores of the SARC-F, MSRA-7, and MSRA-5 were all significantly different between the sarcopenic and nonsarcopenic groups (p ≤ 0.001) in the whole sample and in males and females separately (Table 1).
Sensitivity and specificity
In the entire population, compared with the gold standard of the AWGS, the SARC-F had a sensitivity of 52.5% and a specificity of 96.2%; the MSRA-7 had a sensitivity of 92.4% and a specificity of 51.6%; and the MSRA-5 had a sensitivity of 93.9% and a specificity of 57.3% (Table 2). AUCs of the three scales were very similar (Fig. 1).
Table 2 I still miss predictive values that are very important, because they inform about the probability of having sarcopenia when you have a positive (or negative for the negative predictive value) result in the scales.
Discussion
HF patients often present with fatigue, dyspnoea, and reduced physical strength. These symptoms are not only associated with impaired cardiac pumping, abnormal haemodynamics, and failure of cardiac cells, but also with reduced skeletal muscle function and skeletal muscle cell changes. In recent years, the pathophysiological mechanism of skeletal muscle lesions involved in HF has attracted much attention. Skeletal muscle disease is one of the symptoms of CHF, and its mechanism is via changes in skeletal muscle function, structure, blood flow, metabolism and inflammatory reactions. HF in patients with skeletal muscle lesions includes declining muscle mass and muscle function. Muscle mass decreases mainly because of increased skeletal muscle protein decomposition and reduced synthesis, the transformation of skeletal muscle fibres from type I muscle fibres to type II fast muscle fibres, reduction of capillary distribution density that reduces blood supply, and mitochondrial damage resulting in skeletal muscle loss. In HF patients with sarcopenia, peripheral vascular dysfunction and abnormal skeletal muscle mass and muscle strength lead to decreased exercise tolerance, which is an essential pathophysiological basis for frailty in patients with advanced HF. Patients with HF are prone to frailty, which increases the risk of HF. The frailty-related increase of mortality, disability, and hospitalization is more evident in patients with CHF than those without CHF [7]. Many of the adverse outcomes of frailty may be mediated by sarcopenia, which may be considered the biological substrate for the development of physical frailty and related negative health outcomes [8]. Sarcopenia, frailty, and HF interact to form a vicious cycle [9].
Patients with CHF generally need chest X-ray or chest CT examinations due to the illness. If we measure skeletal muscle mass by CT and so on, not only is the financial burden of the patients increased but the patient’s radioactive ray exposure time is also increased. Most patients with CHF are admitted to the hospital with oedema of the lower limbs and symptoms of chest tightness and asthma, which limit the measurement of skeletal muscle mass by BIA instruments, walking speed and grip strength. The SARC-F, MSRA-7 and MSRA-5 are brief and based on self-reported information. Therefore, they can easily be performed during face-to-face interviews, as self-reported questionnaires, over the telephone, or even through the Internet. The SARC-F has been translated into Korean [10], Chinese [11], Japanese [12, 13], and Spanish [14]. The validity of the SARC-F for screening sarcopenia has also been validated in different ethnic populations. The SARC-F, the early screening tool for sarcopenia, has been widely used in sarcopenia research [15, 16], but few studies involving the SARC-F have been conducted in patients with HF combined with sarcopenia. The MSRA-7 and MSRA-5 are novel screening tools that have not been widely validated in clinical practice. Rossi et al. [6] reported that as pre-screening tool for sarcopenia in the community-dwelling elderly subjects, the sensitivity of the MSRA-7 is 80.4%, and the specificity is 50.5%, while the sensitivity of the MSRA-5 is 80.4% and the specificity is 60.4%. Ming Yang et al. in elderly individuals living in Chinese communities, have found that the sensitivity of the MSRA-7 is 86.9%, and the specificity is 39.6%, while the sensitivity of the MSRA-5 is 90.2%, and the specificity is 70.6% [17]. Our study is the first to validate the accuracy of the SARC-F, MSRA-7 and MSRA-5 in screening for sarcopenia in hospitalized patients with CHF.
The measurement of skeletal muscle mass in our study used the BIA method. In the European Working Group on Sarcopenia in Older People (EWGSOP) [18], the Asia Working Group for Sarcopenia (AWGS) [19], the International Working Group on Sarcopenia (IWGS) [20], and the Foundation for the National Institutes of Health (FNIH) [21] Sarcopenia Project consensus for sarcopenia diagnosis, only the AWGS and EWGSOP recommend the use of BIA to measure skeletal muscle mass. Considering that all of the people we included were Asian, we used the AWGS consensus on sarcopenia as the gold standard. According to the results of Fulster et al.’ s study, the prevalence rate of sarcopenia in patients with mixed heart failure with an average age of 67 was 19.5% [22]. Bekfani et al.[23] indicated that 19.7% of patients with ejection fraction-preserved heart failure had sarcopenia. Kamiya et al. [24] reported that the prevalence of sarcopenia in elderly patients with heart failure was 35.2%. In our study, the prevalence of sarcopenia was 55.8% according to the AWGS diagnostic criteria, 31.0% according to the SARC-F, 73.0% according to the MRSA-7, and 71.3% according to the MRSA-5. Our study showed that the prevalence of sarcopenia in patients with CHF was significantly higher than that in previous studies. The reasons were as follows: ① the enrolled population was HF patients who needed to be hospitalized, and their condition was more serious than that of stable HF patients. ② Our study included 187 patients undergoing long-term haemodialysis (HD), and HF patients undergoing HD perform fewer activities and have more hospitalizations than other patients. ③ Most Chinese individuals do not eat dairy products every day, and some do not even eat dairy products at all. ④ Compared with other previous study populations, our included population needed to restrict liquid intake more strictly, and their dairy intake was lower. These multiple factors led to a significant increase in the prevalence of sarcopenia in our study population.
Compared with the AWGS diagnostic criteria, our study showed that SARC-F screening for sarcopenia in the enrolled population had a low sensitivity (52.5%) and a high specificity (96.2%), while the MRSA-7 and MRSA-5 had a high sensitivity (92.4% for the MSRA-7 and 93.9% for the MSRA-5) and a low specificity (51.6% for the MSRA-7 and 57.3% for the MSRA-5). In our study, the AUCs of the SARC-F, MSRA-7 and MSRA-5 were 0.78, 0.74 and 0.77, respectively, which indicated that the SARC-F, MSRA-7 and MSRA-5 had moderate diagnostic accuracy. In our study, the SARC-F had better specificity, whereas the MRSA-7 and MSRA-5 had higher sensitivity. Therefore, clinicians and researchers should choose the optimal tool according to their specific purpose.
Our study has some limitations. First, we applied BIA instead of the “gold standard” methods (CT, MRI, or DXA) to estimate skeletal muscle mass. Because BIA is a practical and inexpensive method for patients, both the AWGS and EWGSOP recommend using it as an alternative for estimating muscle mass. Second, a small sample size may affect the precision of the accuracy measures obtained. Third, we only compared the SARC-F, MSRA-7, and MSRA-5 questionnaires with commonly used diagnostic criteria in a cross-sectional study. More importantly, the predictive validity of the SARC-F, MSRA-7 and MSRA-5 needs to be tested in longitudinal studies in the future.
Conclusion
The MSRA-7 and MSRA-5 may serve as novel screening tools for sarcopenia in hospitalized patients with CHF. The SARC-F, a classic screening tool, is also suitable for this population. The MSRA-7, MSRA-5, and SARC-F demonstrated similar overall diagnostic accuracy in our study population. The MSRA-7 and MSRA-5 have better sensitivity, whereas the SARC-F has better specificity. These validations of these tools need to be confirmed in longitudinal studies in the future.
References
Herrmann R, Sandek A, von Haehling S et al (2012) Risk stratification in patients with chronic heart failure based on metabolic-immunological, functional and haemodynamic parameters. Int J Cardiol 156:62–68
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Hcart Fail 18:891–975
Loncar G, Springer J, Anker M et al (2016) Cardiac cachexia: hic et nune. J Cachexia Sarcopenia Muscle 7:246–260
Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Cardiology (2018) Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi 46:760–789
Malmstrom TK, Morley JE (2013) SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 14:531–532
Rossi AP, Micciolo R, Rule S et al (2017) Assessing the risk of sarcopenia in the elderly: The Mini Sarcopenia Risk Assessment (MRSA) Questionnaire. J Nutr Health Aging 21:743–749
Testa G, Liguori I, Curcio F et al (2019) Multidimensional frailty evaluation in elderly outpatients with chronic heart failure: a prospective study. Eur J Prev Cardiol 26:1115–1117
Liguori I, Russo G, Ara L et al (2018) Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging 13:913–927
Saitoh M, Ishida J, Doehner W et al (2017) Sarcopenia, cachexia, and muscle performance in heart failure: Review update 2016. Int J Cardiol 238:5–11
Kim S, Kim M, Won CW (2018) Validation of the Korean Version of the SARC-F questionnaire to assess sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc 19:40–45
Woo J, Leung J, Morley JE (2014) Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc 15:630–634
Ida S, Murata K, Nakadachi D et al (2017) Development of a Japanese version of the SARC-F for diabetic patients: an examination of reliability and validity. Aging Clin Exp Res 29:935–942
Ida S, Nakai M, Ito S et al (2017) Association between sarcopenia and mild cognitive impairment using the Japanese version of the SARC-F in elderly patients with diabetes. J Am Med Dir Assoc 18:809.e9–809.e13
Parra-Rodriguez L, Szlejf C, Garcia-Gonzalez AI et al (2016) Cross-cultural adaptation and validation of the Spanish-language version of the SARC-F to assess sarcopenia in Mexican community-dwelling older adults. J Am Med Dir Assoc 17:1142–1146
Wu TY, Liaw CK, Chen FC et al (2016) Sarcopenia screened with SARC-F questionnaire is associated with quality of life and 4-year mortality. J Am Med Dir Assoc 17:1129–1135
Malmstrom TK, Miller DK, Simonsick EM et al (2016) SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 7:28–36
Ming Yang MD, Xiaoyi Hu MSN, Lingling Xie MSN (2019) Comparing mini sarcopenia risk assessment with SARC-F for Screening sarcopenia in community-dwelling older adults. J Am Med Dir Assoc 20:1–5
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Chen LK, Liu LK, Woo J et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Fielding RA, Vellas B, Evans WJ et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc 12:249–256
Studenski SA, Peters KW, Alley DE et al (2014) The FNIH sarcopenia project:rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69:547–558
Fulster S, Taeke M, Sandek A et al (2013) Muscle wasting in patients with chronic heart failure: from the studies investigating co-morbidities Aggravating heart failure (SICA HF). Eur Heart J 34:512–519
Bekfani T, Pellicori P, Morris DA et al (2016) Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle Strength, Exercise capacity and quality of life. Int J Cardiol 222:41–46
Kamiya K, Hamazaki N, Matsuzawa R et al (2017) Sarcopenia: prevalence and prognostic implications in elderly patients with cardiovascular disease. JCSM Clin Rep 2:1–13
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
The research was approved by the medical ethics committee of the Second Affiliated Hospital of Nanjing Medical University.
Statement human right
The ethical committee approved the protocol.
Informed consent
All participants were due informed and they provided consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, W., Lu, M., Wang, X. et al. The role of sarcopenia questionnaires in hospitalized patients with chronic heart failure. Aging Clin Exp Res 33, 339–344 (2021). https://doi.org/10.1007/s40520-020-01561-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01561-9