Abstract
Purpose of review
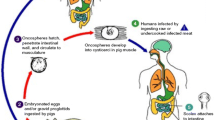
Neurocysticercosis (NCC) results from the localization in the central nervous system (CNS) of larval stages of the cestode parasite Taenia solium. NCC is the most common helminthic parasitic disease of the nervous system and the main cause of acquired epilepsy. This literature review presents an update in NCC diagnostic techniques, including neuroimaging, immunological assays, molecular assays, and clinical manifestations.
Recent findings
NCC can cause a wide array of neurological manifestations, most of them being non-pathognomonic. Diagnosis of this infection represents a challenge due to its nonspecific symptoms and limited resources in endemic regions. Diagnoses by neuroimaging and immunological assessment are the current criteria used to diagnose NCC.
Summary
Current diagnostic techniques continue to be non-affordable and non-available in most endemic regions. The development of novel and affordable standardized diagnostic techniques is of urgent need in order to determine the actual global burden caused by NCC.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Garcia HH, Gilman R, Martinez M, Tsang VC, Pilcher JB, Herrera G, et al. Cysticercosis as a major cause of epilepsy in Peru. Lancet. 1993;341(8839):197–200.
Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4(10):653–61.
Garcia HH, Gonzalez AE, Rodriguez S, Tsang VC, Pretell EJ, Gonzales I, et al. Neurocysticercosis: unraveling the nature of the single cysticercal granuloma. Neurology. 2010;75(7):654–8.
Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol. 2011;7(10):584–94.
Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8(9):871–4.
Chavarria A, Fleury A, Garcia E, Marquez C, Fragoso G, Sciutto E. Relationship between the clinical heterogeneity of neurocysticercosis and the immune-inflammatory profiles. Clin Immunol. 2005;116(3):271–8.
Waterhouse R. Cysticercus cellulosae in the central nervous system: with an account of two cases. QJM: Int J Med. 1913;os6(4):469–85.
Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VC. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. Cysticercosis Working Group in Peru. J Infect Dis. 1997;175(2):486–9.
Grogl M, Estrada JJ, MacDonald G, Kuhn RE. Antigen-antibody analyses in neurocysticercosis. J Parasitol. 1985;71(4):433–42.
Diwan AR, Coker-Vann M, Brown P, Subianto DB, Yolken R, Desowitz R, et al. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibody to cysticerci of Taenia solium. Am J Trop Med Hyg. 1982;31(2):364–9.
Gottstein B, Tsang VC, Schantz PM. Demonstration of species-specific and cross-reactive components of Taenia solium metacestode antigens. Am J Trop Med Hyg. 1986;35(2):308–13.
Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. 1989;159(1):50–9.
• Gomez-Morales MA, Garate T, Blocher J, Devleesschauwer B, Smit GSA, Schmidt V, et al. Present status of laboratory diagnosis of human taeniosis/cysticercosis in Europe. Eur J Clin Microbiol Infect Dis. 2017;36(11):2029–40. This study mentions the different tests used in European laboratories for human taeniosis/cysticercosis detection and to obtain preliminary data on the number of diagnosed taeniosis/CC cases.
•• Del Brutto OH, Nash TE, White AC Jr, Rajshekhar V, Wilkins PP, Singh G, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202–10. This article presents a revised and updated set of diagnostic criteria in order to provide a more uniform diagnostic tool for clinicans.
Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5(5):e1152.
Singhi P. Neurocysticercosis. Ther Adv Neurol Disord. 2011;4(2):67–81.
Sharma S, Modi M, Lal V, Prabhakar S, Bhardwaj A, Sehgal R. Reversible dementia as a presenting manifestation of racemose neurocysticercosis. Ann Indian Acad Neurol. 2013;16(1):88–90.
•• Jensen TO, Post JJ. Intraventricular neurocysticercosis: presentation, diagnosis and management. Asian Pac J Trop Med. 2016;9(8):815–8. This article focuses on the clinical, serological, and neuroimaging diagnostics of intraventricular neurocysticercosis.
• Webb CM, White AC Jr. Update on the diagnosis and management of neurocysticercosis. Curr Infect Dis Rep. 2016;18(12):44. This review discusses an update on the diagnosis and treatments used for different stages of NCC, focusing on emerging data that are demonstrating that the optimal management approach varies with stage.
Del Brutto OH. Diagnostic criteria for neurocysticercosis, revisited. Pathog Glob Health. 2012;106(5):299–304.
Nash TE, Garcia HH, Rajshekhar V, Del Brutto OH. Clinical cysticercosis: diagnosis and treatment. In: Murrell KD, editor. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/Cysticercosis. Paris, France. 2005. p. 11–25.
•• Del Brutto OH, Arroyo G, Del Brutto VJ, Zambrano M, Garcia HH. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: a large-scale, computed tomography-based population study in rural Ecuador. Epilepsia. 2017;58(11):1955–61. This article focuses on the different uses of CT scans as a diagnostic tool for calcified NCC and its association with epilepsy.
Suss RA, Maravilla KR, Thompson J. MR imaging of intracranial cysticercosis: comparison with CT and anatomopathologic features. AJNR Am J Neuroradiol. 1986;7(2):235–42.
Del Brutto OH, Lama J, Zambrano M, Del Brutto VJ. Neurocysticercosis is a neglected microbleed mimic. A cautionary note for stroke neurologists. Eur Neurol. 2014;72(5–6):306–8.
Carrillo Mezo R, Lara Garcia J, Arroyo M, Fleury A. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop. 2015;152:60–5.
Hernández RDD, Durán BB, Lujambio PS. Magnetic Resonance Imaging in Neurocysticercosis. Topics in Magnetic Resonance Imaging. 2014;23(3):191. https://doi.org/10.1097/RMR.0000000000000026.
Mont’Alverne Filho FE, Machado Ldos R, Lucato LT, Leite CC. The role of 3D volumetric MR sequences in diagnosing intraventricular neurocysticercosis: preliminar results. Arq Neuropsiquiatr. 2011;69(1):74–8.
Do Amaral LL, Ferreira RM, da Rocha AJ, Ferreira NP. Neurocysticercosis: evaluation with advanced magnetic resonance techniques and atypical forms. Top Magn Reson Imaging. 2005;16(2):127–44.
Carpio A, Romo ML. State of the art in neurocysticercosis: imaging and epidemiology. Asian Pac J Trop Med. 2016;9(8):821–2.
• Singh AK, Garg RK, Rizvi I, Malhotra HS, Kumar N, Gupta RK. Clinical and neuroimaging predictors of seizure recurrence in solitary calcified neurocysticercosis: a prospective observational study. Epilepsy Res. 2017;137:78–83. This article refers to the different uses of neuroimaging and clinical criteria to diagnose NCC.
Fernandez-Bouzas A, Harmony T, Fernandez T, Ricardo-Garcell J, Casian G, Sanchez-Conde R. Cerebral blood flow and sources of abnormal EEG activity (VARETA) in neurocysticercosis. Clin Neurophysiol. 2001;112(12):2281–7.
Fernandez-Bouzas A, Harmony T, Bosch J, Aubert E, Fernandez T, Valdes P, et al. Sources of abnormal EEG activity in the presence of brain lesions. Clin EEG. 1999;30(2):46–52.
Dorny P, Brandt J, Zoli A, Geerts S. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 2003;87(1):79–86.
Dorny P, Brandt J, Geerts S. Detection and diagnosis. In: Murrell KD, Weltgesundheitsorganisation, & FAO, editor. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/Cysticercosis. Paris, OIE. 2005. p. 45–55.
Bueno EC, Vaz AJ, Machado LD, Livramento JA. Neurocysticercosis: detection of IgG, IgA and IgE antibodies in cerebrospinal fluid, serum and saliva samples by ELISA with Taenia solium and Taenia crassiceps antigens. Arq Neuropsiquiatr. 2000;58(1):18–24.
Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 2010;26(3):137–44.
Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health. 2012;106(5):286–98.
Salazar-Anton F, Lindh J. Taenia solium: a two-dimensional Western blotting method combined with the use of an EST-library for the identification of immunogenic proteins recognized by sera from neurocysticercosis patients. Exp Parasitol. 2011;128(4):371–6.
Scheel CM, Khan A, Hancock K, Garcia HH, Gonzalez AE, Gilman RH, et al. Serodiagnosis of neurocysticercosis using synthetic 8-KD proteins: comparison of assay formats. Am J Trop Med Hyg. 2005;73(4):771–6.
Hancock K, Khan A, Williams FB, Yushak ML, Pattabhi S, Noh J, et al. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J Clin Microbiol. 2003;41(6):2577–86.
Noh J, Rodriguez S, Lee YM, Handali S, Gonzalez AE, Gilman RH, et al. Recombinant protein- and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol. 2014;52(5):1429–34.
Zimic M, Pajuelo M, Rueda D, Lopez C, Arana Y, Castillo Y, et al. Utility of a protein fraction with cathepsin L-like activity purified from cysticercus fluid of Taenia solium in the diagnosis of human cysticercosis. Am J Trop Med Hyg. 2009;80(6):964–70.
Baig S, Damian RT, Molinari JL, Tato P, Morales-Montor J, Welch M, et al. Purification and characterization of a metacestode cysteine proteinase from Taenia solium involved in the breakdown of human IgG. Parasitology. 2005;131(Pt 3):411–6.
Li AH, Moon SU, Park YK, Na BK, Hwang MG, Oh CM, et al. Identification and characterization of a cathepsin L-like cysteine protease from Taenia solium metacestode. Vet Parasitol. 2006;141(3–4):251–9.
Harmsen MM, Cornelissen JB, Buijs HE, Boersma WJ, Jeurissen SH, van Milligen FJ. Identification of a novel Fasciola hepatica cathepsin L protease containing protective epitopes within the propeptide. Int J Parasitol. 2004;34(6):675–82.
Hernández M, Beltrán C, Garcı́a E, Fragoso G, Gevorkian G, Fleury A, et al. Cysticercosis: towards the design of a diagnostic kit based on synthetic peptides. Immunol Lett. 2000;71(1):13–7.
Fleury A, Beltran C, Ferrer E, Garate T, Harrison LJ, Parkhouse RM, et al. Application of synthetic peptides to the diagnosis of neurocysticercosis. Tropical Med Int Health. 2003;8(12):1124–30.
Hell RC, Amim P, de Andrade HM, de Avila RA, Felicori L, Oliveira AG, et al. Immunodiagnosis of human neurocysticercosis using a synthetic peptide selected by phage-display. Clin Immunol. 2009;131(1):129–38.
Paredes A, Saenz P, Marzal MW, Orrego MA, Castillo Y, Rivera A, et al. Anti-Taenia solium monoclonal antibodies for the detection of parasite antigens in body fluids from patients with neurocysticercosis. Exp Parasitol. 2016;166:37–43.
Zea-Vera A, Cordova EG, Rodriguez S, Gonzales I, Pretell EJ, Castillo Y, et al. Parasite antigen in serum predicts the presence of viable brain parasites in patients with apparently calcified cysticercosis only. Clin Infect Dis. 2013;57(7):e154–9.
Erhart A, Dorny P, Van De N, Vien HV, Thach DC, Toan ND, et al. Taenia solium cysticercosis in a village in northern Viet Nam: seroprevalence study using an ELISA for detecting circulating antigen. Trans R Soc Trop Med Hyg. 2002;96(3):270–2.
Fleury A, Hernandez M, Avila M, Cardenas G, Bobes RJ, Huerta M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78(9):970–4.
Castillo Y, Rodriguez S, Garcia HH, Brandt J, Van Hul A, Silva M, et al. Urine antigen detection for the diagnosis of human neurocysticercosis. Am J Trop Med Hyg. 2009;80(3):379–83.
Gonzalez AE, Bustos JA, Garcia HH, Rodriguez S, Zimic M, Castillo Y, et al. Successful antiparasitic treatment for cysticercosis is associated with a fast and marked reduction of circulating antigen levels in a naturally infected pig model. Am J Trop Med Hyg. 2015;93(6):1305–10.
Garcia HH, Parkhouse RM, Gilman RH, Montenegro T, Bernal T, Martinez SM, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2000;94(6):673–6.
Ribeiro Vda S, Araujo TG, Gonzaga HT, Nascimento R, Goulart LR, Costa-Cruz JM. Development of specific scFv antibodies to detect neurocysticercosis antigens and potential applications in immunodiagnosis. Immunol Lett. 2013;156(1–2):59–67.
Carpio A, Campoverde A, Romo ML, Garcia L, Piedra LM, Pacurucu M, et al. Validity of a PCR assay in CSF for the diagnosis of neurocysticercosis. Neurol Neurophysiol Neurosci. 2017;4(2):e324.
Hernandez M, Gonzalez LM, Fleury A, Saenz B, Parkhouse RM, Harrison LJ, et al. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol. 2008;102(4):317–23.
Almeida CR, Ojopi EP, Nunes CM, Machado LR, Takayanagui OM, Livramento JA, et al. Taenia solium DNA is present in the cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):307–10.
Hoban DJ, Witwicki E, Hammond GW. Bacterial antigen detection in cerebrospinal fluid of patients with meningitis. Diagn Microbiol Infect Dis. 1985;3(5):373–9.
Cárdenas G, Valdez R, Sáenz B, Bottasso O, Fragoso G, Sciutto E, et al. Impact of Taenia solium neurocysticercosis upon endocrine status and its relation with immuno-inflammatory parameters. Int J Parasitol. 2012;42(2):171–6.
Sahu PS, Parija SC, Narayan SK, Kumar D. Evaluation of an IgG-ELISA strategy using Taenia solium metacestode somatic and excretory-secretory antigens for diagnosis of neurocysticercosis revealing biological stage of the larvae. Acta Trop. 2009;110(1):38–45.
Nunes D, Gonzaga H, Ribeiro V, Cunha-Júnior J, Costa-Cruz J. Usefulness of gel filtration fraction as potential biomarker for neurocysticercosis in serum: towards a new diagnostic tool. Parasitology. 2017;144(4):426–35.
Gutierrez-Loli R, Orrego MA, Sevillano-Quispe OG, Herrera-Arrasco L, Guerra-Giraldez C. MicroRNAs in Taenia solium neurocysticercosis: insights as promising agents in host-parasite interaction and their potential as biomarkers. Front Microbiol. 2017;8:1905.
Singh A, Prasad KN, Singh AK, Singh SK, Gupta KK, Paliwal VK, et al. Human glutathione S-transferase enzyme gene polymorphisms and their association with neurocysticercosis. Mol Neurobiol. 2017;54(4):2843–51.
Lachuriya G, Garg RK, Jain A, Malhotra HS, Singh AK, Jain B, et al. Toll-like receptor-4 polymorphisms and serum matrix metalloproteinase-9 in newly diagnosed patients with calcified neurocysticercosis and seizures. Medicine. 2016;95(17):e3288.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nicholas A. Gadea declares that he has no conflict of interest. Maria Mercedes Rueda declares that she has no conflict of interest. Gabriela Matamoros declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neglected Tropical Diseases
Rights and permissions
About this article
Cite this article
Gadea, N.A., Matamoros, G. & Rueda, M.M. Recent Advances in the Diagnosis of Neurocysticercosis. Curr Treat Options Infect Dis 10, 410–420 (2018). https://doi.org/10.1007/s40506-018-0173-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-018-0173-9