Abstract
Purpose of Review
Periodontitis, one of the most prevalent diseases in the world, is caused by the accumulation of dysbiotic microbial biofilm on the teeth leading to chronic inflammation of the tissues surrounding the teeth. Type 2 diabetes mellitus (T2DM), obesity, chronic stress, and smoking are some of the risk factors for the disease. A high-carbohydrate diet also increases the risk of periodontal inflammation. Modifying diet and nutrition could serve as a preventive and therapeutic tool to target multiple risk factors simultaneously.
Recent Findings
Emerging evidence shows that the ketogenic diet induces hormetic stress and switches on various cell-protective anti-inflammatory and antioxidant mechanisms. The ketogenic diet also improves mitochondrial function, DNA repair, and autophagy. The diet can effectively treat periodontitis risk factors such as T2DM and obesity. By restricting carbohydrates, the diet improves glycaemic control in T2DM patients and can effectively produce fat loss and reduce BMI (body-mass index) in obese patients. Poor long-term compliance and high cost are the drawbacks of the diet and the potential of the diet to increase cardiovascular disease risk needs further investigation.
Summary
Taken together, ketogenic diets, through various mechanisms reduce inflammation, mitigate oxidative stress, improve metabolic health, and can be used as a therapeutic tool to treat periodontal inflammation. Since robust scientific evidence for the ketogenic diet is currently scarce, future research should study the diet's efficacy, effectiveness, and safety in managing periodontal inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Let food be thy medicine and medicine be thy food.”
- Hippocrates
An optimal diet is the cornerstone of any healthy lifestyle as nutrition plays a major role in human health and disease. In modern times, several chronic non-communicable diseases—termed ‘diseases of civilisation’—cardiovascular disease (CVD), neurodegenerative disease, type 2 Diabetes mellitus (T2DM), metabolic syndrome, and cancer are the leading causes of death and disability and form the crux of our healthcare burden. Many chronic non-communicable diseases have integral links to a sub-optimal diet, nutrition, and lifestyle [1, 2].
Periodontitis—the sixth most prevalent disease in the world [3,4,5]—is a chronic non-communicable disease that manifests as inflammation of the soft tissues surrounding the tooth. The dysbiosis of the oral microbial biofilm initiates a chain of inflammatory events that culminate in chronic inflammation of the periodontium. The development and progression of periodontal inflammation are influenced by a multitude of other prevalent chronic diseases and conditions like T2DM, obesity, and chronic stress [6]. There is also abundant evidence that periodontitis is influenced by diet and nutrition [7, 8]. The management of periodontitis mainly focuses on removing dysbiotic biofilm from the teeth and enforcing optimal oral hygiene in the patient. Since periodontitis is affected by numerous factors, it is imperative to have a simple and effective strategy that can simultaneously target multiple risk factors for the disease. Modifying diet and nutrition could be one such strategy.
Macronutrients (carbohydrates, proteins, and fats); micronutrients (vitamins and minerals); and dietary fibre are the major constituents of any diet. An optimal diet must ensure a proper balance between these constituents without creating excess or deficits, provide the required calorie intake and facilitate optimal health and function. Over the years, several diets have emerged that attempt to prevent disease and optimize human health and well-being. The ketogenic diet is one such diet that originated in the 1920s, mainly to treat epilepsy. Russel Wilder coined the term ‘Ketogenic diet’ and proposed its use as therapy in epileptic patients. Although the diet was very effective in treating seizures, it lost popularity when more potent drugs were introduced [9••].
Since the last two decades, ketogenic diets have regained popularity as a dietary measure to treat and prevent many chronic diseases and to enhance health and well-being. The ketogenic diet advocates for consuming a high percentage of fats and a very low percentage of carbohydrates and aims to shift the body to an alternative source of energy. When on non-ketogenic diets, carbohydrates serve as the primary energy source in the form of glucose. But on a ketogenic diet, since carbohydrates are severely restricted, the body shifts to using fat for energy in the form of ketone bodies. There are several types of ketogenic diets with varying proportions of fat and carbohydrates (Fig. 1).
Types of Ketogenic Diets and their macronutrient proportions compared to the USDA dietary guidelines. (KD- Ketogenic diet; MCT- Medium-chain Triglyceride; LGIT- Low glycaemic index treatment; USDA- United States Department of Agriculture). The USDA dietary guidelines [10]. (Original figure designed in BioRender software)
Emerging evidence in scientific literature highlights ketogenic diets' anti-inflammatory and antioxidant properties and their potential in managing chronic diseases. Recent studies suggest that ketogenic diets can also have a positive effect on periodontitis. In this review, we explore the scientific literature to discuss the effects of ketogenic diets, their underlying mechanisms, merits and demerits, and their potential in preventing and managing periodontal inflammation.
Ketogenic Diets Alter Energy Metabolism
Glycolysis (breakdown of glucose into pyruvate) and Lipolysis (breakdown of triglycerides into free fatty acids) are two major biochemical processes of cellular respiration that sustain life. In the presence of adequate carbohydrates, the body relies on glucose to be its primary source of fuel. Glycolysis in the cytoplasm, the citric acid cycle and oxidative phosphorylation in the mitochondria together produce energy from glucose in the form of Adenosine Triphosphate (ATP) [11]. However, when there is a deficiency of carbohydrates, the body adapts to using fatty acids for energy [12••].
A ketogenic diet is a high-fat diet that is low in carbohydrates and induces a metabolic state of ketosis, in which the body adopts ketone bodies that are produced from free fatty acids as an alternative fuel source instead of glucose [9••] (Fig. 2). Since the diet reduces the intake of carbohydrates, there is a smaller rise in blood glucose levels and less insulin is secreted. Insulin is a hormone that promotes glucose uptake and utilization by cells and inhibits lipolysis and ketogenesis (the synthesis of ketone bodies from acetyl-CoA) [12, 13]. Therefore, the ketogenic diet, by restricting carbohydrates and decreasing insulin levels, reduces the reliance on glucose as an energy source; and promotes lipolysis and ketogenesis to meet the energy requirements of the body.
Ketogenesis during starvation or ketogenic diets: Low levels of insulin during starvation or ketogenic diets cause the breakdown of triglycerides into free fatty acids (FFA). In the liver cell, FFA is β-oxidised to acetyl-CoA. However, acetyl CoA is unable to enter the Krebs cycle as oxaloacetate is depleted because of gluconeogenesis. Hence the acetyl-CoA is converted to ketone bodies (mainly β-hydroxybutyrate) and is transported to extra-hepatic cells where they can be converted back to acetyl-CoA. Since there is no gluconeogenesis happening in extra-hepatic cells, acetyl-CoA can enter the Krebs cycle to produce adenosine triphosphate (ATP). (Original figure designed in BioRender software)
Lipolysis occurs mainly in adipose tissue where hormone-sensitive lipase and other enzymes convert triglycerides into glycerol and fatty acids. These free fatty acids are transported to various tissues by blood. They are oxidised (β-oxidation) in the mitochondria to produce acetyl-CoA, which can enter the citric acid cycle to generate ATP, NADH, and FADH2 (electron carriers that go on to participate in oxidative phosphorylation). However, during prolonged carbohydrate restriction or starvation, citric acid cycle intermediates like oxaloacetate are exhausted in the liver due to the gluconeogenesis pathway and hence, the acetyl-CoA from fatty acid oxidation is unable to enter the citric acid cycle [12••]. Instead, the acetyl-CoA is converted to ketone bodies. Ketogenesis occurs mainly in the liver mitochondria, where excess accumulating acetyl-CoA is converted into the ketone bodies: acetoacetate, β-hydroxybutyrate, and acetone. Most acetoacetate is reduced to β-hydroxybutyrate whereas acetone is spontaneously exhaled through the lungs. Hence, β-hydroxybutyrate becomes the major ketone body in the blood [12••].
Ketone bodies are transported to other extra-hepatic tissues by blood, where they can either be used up for the energy needs of the cells or fatty acid synthesis [14, 15]. In the mitochondria of extra-hepatic cells, β-hydroxybutyrate is converted back to acetoacetate and eventually to acetyl-CoA by a host of mitochondrial enzymes. This acetyl-CoA can enter the citric acid cycle to produce the intermediates required for the electron transport chain and oxidative phosphorylation for energy production. Thereby, ketone bodies substitute for glucose and serve as an alternate source of energy during ketogenic diets. Ketone bodies can be metabolized faster, can bypass glycolysis, and directly enter the Kreb’s cycle, and can be utilized by all extra-hepatic tissues including the brain and the heart as a more efficient source of energy [12, 16,17,18,19]—Ketone bodies can cross the blood–brain barrier to supply energy to the brain when on a ketogenic diet [18, 20]. This adaptive shift of the body to use an ancillary source of energy is critical for survival and brain function during extended periods of starvation and scarcity of carbohydrates.
The Complexity of Periodontal Inflammation
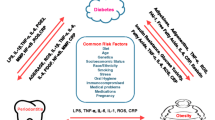
Due to the inherent nature of a tooth (non-shedding hard tissue that is moist and exposed to the external environment), it is prone to microbial biofilm formation on its surface. The oral microbiota deposit as biofilm communities on the teeth. Dysbiosis of the microbial biofilm community elicits an altered immune response from the body leading to prolonged inflammation of the supporting tissues of the teeth [21]. This inflammation of the periodontium (supporting tissues around the teeth) is termed periodontitis. The consequence of unresolved periodontal inflammation is gradual loss of tooth-supporting tissues and tooth loss. Several factors contribute towards the dysbiosis of microbial biofilm and periodontal inflammation: poor oral hygiene, diet, smoking, systemic conditions like obesity, stress, diabetes, and other pro-inflammatory diseases [6]. Increase in pro-inflammatory cytokines like interleukin-1 (IL-1), interleukin-6 (IL-6) or tumour necrosis factor-α (TNF- α); activation of signalling pathways such as nuclear factor-κB (NF-κB) and NOD-like receptor protein 3 (NLRP3) inflammasome are characteristic of periodontitis and together function to activate and augment the immune response of the host [22,23,24,25,26].
The host immune-inflammatory response against the dysbiotic biofilm causes large numbers of polymorphonuclear neutrophils (PMNs) to migrate towards the gingival sulcus. PMNs are capable of phagocytosis and constitute the immune system’s first line of defence. Excessive oxidative killing of bacteria by the PMNs after phagocytosis results in increased production and accumulation of ROS in the periodontal tissues leading to oxidative stress [27]. Oxidative stress plays a major role in the pathogenesis of periodontal inflammation [27]. Oxidative stress causes damage to various cellular organelles including mitochondria and DNA and leads progression of periodontal inflammation [27]. Patients with periodontitis have two times greater 8-hydroxy-2'-deoxyguanosine (a marker of oxidative stress) in saliva when compared to healthy subjects [28]. A 2023 meta-analysis revealed that periodontitis patients have significantly higher oxidative stress along with a lower antioxidant capacity than healthy subjects and that oxidative stress is an important feature in progressive periodontitis [29].
Obesity increases the risk for periodontitis. Obesity results in systemic hyper-inflammation and oxidative stress and exhibits a bi-directional relationship with periodontitis [30]. Elevated serum C-reactive protein (CRP) is a common factor in obesity and severe periodontitis [31, 32]. A 2017 systematic review revealed that obese individuals were more prone to periodontitis possibly due to the low-grade systemic inflammation and oxidative stress that is seen in obesity [33]. A more recent meta-analysis concluded that periodontitis and obesity are positively associated irrespective of age [34].
The chronic inflammatory state that stems from obesity leads to insulin resistance [35]. The adipose tissue produces several pro-inflammatory cytokines and adipokines that disrupt various insulin signalling pathways leading to insulin resistance in adipocytes and peripheral tissues [36]. Insulin resistance leads to oxidative stress in the pancreatic β-cells resulting in impaired insulin secretion [36]. Insulin resistance is an important feature of Type-2 Diabetes mellitus (T2DM) which is a well-known and well-established risk factor for periodontitis with a bi-directional relationship [37,38,39,40,41].
Diet plays an integral role in the pathogenesis of periodontal inflammation and more specifically, a diet that is high in carbohydrates is linked with chronic inflammation [42•]. A recent observational study on humans found that the total dietary carbohydrate intake is positively associated with inflammation [43]. In mice, greater inflammation was seen on a high carbohydrate than on a high-fat diet [44]. Excessive consumption of refined carbohydrates induces neuroinflammation in mice [45]. Excessive dietary carbohydrates increase the risk of periodontitis as glycaemia leads to oxidative stress and the accumulation of advanced glycation end products that result in a hyper-inflammatory state [46,47,48]. Dietary carbohydrates can also impact the subgingival microbiota and lead to poor oral health outcomes; higher carbohydrate consumption was associated with reduced diversity of subgingival microflora in postmenopausal women [49]. A high-carbohydrate diet is associated with a higher prevalence of Fusobacteria species (which plays an important role in dental plaque biofilm maturation) [50]. A 2022 systematic review concluded that a sugar-rich diet decreased oral microbial diversity [51].
Overall, periodontitis is complex; associated with many modifiable and non-modifiable risk factors; manifests as prolonged local inflammation around the teeth; and is accompanied by oxidative stress and elevated levels of pro-inflammatory cytokines (Fig. 3). Hence, an optimal strategy for preventing and managing periodontitis—along with removal of dysbiotic biofilm and enforcement of adequate oral hygiene—would include the use of anti-inflammatory and antioxidant therapies that can simultaneously target multiple risk factors of the disease.
The complexity of periodontal inflammation and its risk factors: Excessive neutrophil infiltration of gingival tissues causes connective tissue destruction resulting in deepening of the periodontal pocket. Phagocytosis and oxidative killing by these neutrophils cause oxidative stress. The deepened periodontal pocket facilitates the down growth of the biofilm. Eventually, alveolar bone is resorbed to prevent the inflammatory response from reaching the bone. If the inflammation is unresolved, the bone loss continues and leads to loss of tooth support, culminating in tooth loss. (Original figure designed in BioRender software)
Ketogenic Diets are Antioxidant and Anti-Inflammatory
Apart from being a supplementary energy source for the body, ketone bodies induce numerous other alterations in systemic physiology. Initially, the response to ketone-fuelled functioning is an increase in reactive oxygen species (ROS) in the mitochondria, an increase in NAD+/NADH ratio, a reduction in AMP/ATP ratio and oxidative stress [12, 19, 52,53,54,55,56]. There is also an increase in pro-inflammatory cytokines such as IL-1, IL-6 and TNF- α [12, 54]. This acute oxidative stress and inflammation leads to hormesis that activates protective cellular responses [12••]. Hormesis is a phenomenon where a low-strength/low-dose stress factor induces an adaptive defence response in the body [57]. The hormetic stress of the ketogenic diet activates antioxidant and anti-inflammatory mechanisms [12, 14, 58,59,60].
Nuclear factor E2-related factor 2 (Nrf2) transcription factor is responsive to oxidative stress and switches on cell-protective genes responsible for producing antioxidant enzymes through the Nrf2/ARE (Antioxidant Response Element) pathway [61, 62]. Rats fed with a KD showed an initial increase in hydrogen peroxide levels followed by a reduction below control levels after 3 weeks. There was also an increased nuclear accumulation of Nrf2 in the liver and hippocampus after 3 weeks suggesting that a ketogenic diet activates the antioxidant and cytoprotective Nrf2 pathway after an acute phase of mild oxidative stress [63]. In rats with spinal cord injury, KD activated the Nrf2 pathway, suppressed the pro-inflammatory nuclear factor-κB (NF-κB) signalling pathway, and reduced the expression of pro-inflammatory cytokines TNF-α, IL-1β, and IFN-γ (Interferon-γ) [64]. Overall, the KD has been shown to have a prolonged antioxidant and anti-inflammatory effect due to the activation of the Nrf2 pathway [63,64,65].
Sirtuins (SIRTs) 1 and 3 are NAD+-dependent histone deacetylases involved in ageing, apoptosis, and autophagy (the process of lysosomal degradation of defective cellular components and promote cell homeostasis, differentiation, and survival [66]). The increase in NAD+/NADH ratio during KD activates the Sirtuin1 (SIRT1) enzyme that induces increased expression of antioxidant and anti-inflammatory genes and supports DNA repair and autophagy [67]. Rats on a KD showed increased levels of SIRT1 enzymes in serum and white adipose tissue [68]. Mice injected with exogenous ketone bodies (acetoacetate and β-hydroxybutyrate) showed increased expression of SIRT3 leading to reduced oxidative stress and improved mitochondrial function [69].
The increase in the AMP/ATP ratio during a KD upregulates AMP-activated kinases (AMPK) that increase cytoprotective antioxidant and anti-inflammatory functions, DNA repair, and autophagy [12, 70]. Intracellular oxidative stress leads to the endoplasmic reticulum (ER) stress that induces NOD-like receptor protein 3 (NLRP-3) inflammasome which activates the pro-inflammatory cytokine IL-1β. Rats injected with β-hydroxybutyrate in a fasted state showed lower ER stress, lower NLRP-3 inflammasome formation, and increased expression of antioxidants manganese superoxide dismutase and catalase through the activation of AMPK [71]. In epileptic mice, β-hydroxybutyrate reduced neuronal damage through the activation of AMPK and other anti-oxidative mechanisms [72].
Taken together, ketogenic diets act as a hormetic stress factor that upregulates adaptive antioxidant and anti-inflammatory cellular responses by activating the Nrf2/ARE pathway, SIRT1 and SIRT3, and AMPK. KD also contributes to improved mitochondrial function, DNA repair, and autophagy.
Ketogenic Diets Improve Metabolic Health
Insulin is an anabolic hormone that promotes cellular glucose utilization, inhibits lipolysis, and is a master hormone that regulates metabolic health [73]. Ketogenic diets restrict dietary carbohydrates resulting in low circulating insulin levels. Low insulin levels promote lipolysis, ketogenesis and fat loss. Low-calorie ketogenic diets result in a significantly greater reduction in body fat and weight compared to low-calorie non-ketogenic diets [74]. A 2020 systematic review and meta-analysis concluded that very low-calorie ketogenic diets were able to produce weight loss that was stable for up to 2 years of follow-up, with reductions in waist circumference, body mass index (BMI), and triglyceride levels [75]. Hence, ketogenic diets can be used as a therapeutic tool to manage obesity [76].
Lower post-prandial insulin levels during ketogenic diets can prevent hyperinsulinemia, reduce insulin resistance, and increase insulin sensitivity of peripheral tissues [12, 77]. A 2020 systematic review and meta-analysis revealed that ketogenic diets produced greater improvements in glycaemic control in terms of glycated haemoglobin (HbA1C) levels when compared to low-fat diets in obese patients with T2DM. The study concluded that ketogenic diets more effectively improved metabolic health than low-fat diets. Several clinical trials show greater reduction in fasting blood glucose levels with ketogenic diets when compared to non-ketogenic diets [12••]. Hence ketogenic diets hold the potential to combat insulin resistance and thereby can prevent and manage T2DM.
Clinical Relevance and Drawbacks
Periodontal inflammation is closely associated with diet and nutrition. The roles of various macro and micronutrients in periodontitis have been described extensively in the scientific literature [42, 48, 78]. Subjects who scored high on the Healthy Eating Index (HEI) had less risk for periodontal disease [79]. A recent pilot study compared a high-carbohydrate diet to an oral health-optimized low-carbohydrate diet (also rich in omega-3 fatty acids, vitamin C, and vitamin D) and found that the latter reduced the load of periodontal pathogens in supragingival plaque [80].
Ketogenic diets target multiple risk factors for periodontitis—the diet restricts carbohydrates; reduces insulin resistance and enhances insulin sensitivity; improves glycaemic control and mitigates T2DM; reduces body fat, body mass index (BMI), and ameliorates obesity; downregulates pro-inflammatory markers and upregulates anti-inflammatory and antioxidant defence mechanisms of cells. Hence, ketogenic diets hold preventive and therapeutic potential against periodontal inflammation.
However, the scientific literature currently shows very few studies evaluating ketogenic or similar diets against periodontal inflammation. In a recent study, a high-fat, low-carbohydrate diet reduced IL-6 and CRP levels in T2DM patients compared to a high-carbohydrate diet [81]. Low-carbohydrate, high-fibre diet was associated with a decreased risk for periodontitis [82]. A 2017 pilot study demonstrated that a diet low in carbohydrates and high in omega-3 fatty acids can significantly reduce gingival and periodontal inflammation [83]. Another recent pilot study evaluated a ketogenic diet and found that the diet did not significantly improve the periodontal clinical parameters [84]. In contrast, a 2024 scoping review concluded that ketogenic diets might have beneficial anti-inflammatory and antioxidant effects on periodontal inflammation [85].
There is also scientific evidence that a high-fat diet increases the risk of periodontitis. High-fat diets are tied to chronic low-grade systemic inflammation and various diseases [86]. High-fat diets induce periodontal inflammation in mice while increasing the prevalence of periodontal pathogens, gingival inflammation, and alveolar bone loss [87, 88]. A high-fat diet also caused the progression of apical periodontitis lesions when compared to a control diet [89]. A 2023 systematic review concluded that a high-fat diet (and other types of unbalanced diets like high-carbohydrate diets) could increase the risk for periodontitis [8]. However, these studies evaluating high-fat diets do not standardise carbohydrate intake. Ketogenic diets are not merely high-fat diets but also very low in dietary carbohydrates. The lack of sufficient carbohydrates in ketogenic diets leads to metabolic flexibility where the body is forced to use fat for fuel (ketone bodies produced from fatty acids). This creates a hormetic stress response that triggers anti-inflammatory and antioxidant defences of the body. Hence ketogenic diets are distinct, and the lack of robust clinical evidence dictates the need for well-designed studies to evaluate its potential in the future.
There could be several limitations and drawbacks to the ketogenic diet. First, ketogenic diets are severely restrictive in the choice of food items and therefore could be difficult to comply with in the long term. Also, complying with such a diet might not be economically feasible for most people. Second, although dietary cholesterol does not affect serum cholesterol or cardiovascular disease risk [90,91,92,93], several studies and case reports show that a ketogenic diet leads to an increase in low-density lipoprotein (LDL) levels in the blood [94,95,96]. Although controversial, elevated serum LDL cholesterol levels are associated with an increased risk for atherosclerotic cardiovascular disease (ASCVD) [97,98,99]. However, ketogenic diets are known to increase large-buoyant LDL particles that are cardioprotective as opposed to the small-dense LDL particles that are associated with cardiovascular disease [12, 100]. Hence, the potential of ketogenic diets to increase cardiovascular disease needs further investigation.
Concluding Remarks and Future Directions
Despite the drawbacks, ketogenic diets seem to have several advantages and can potentially mitigate periodontal inflammation (Fig. 4). Ketogenic diets alter energy metabolism by restricting carbohydrates and inducing a state of ketosis where ketone bodies are used as the energy source for the body. This hormetic stress leads to the activation and upregulation of anti-inflammatory and antioxidant mechanisms that are protective against periodontal inflammation—The mere restriction of carbohydrates by itself can promote periodontal health since a high-carbohydrate diet increases the risk of periodontitis—The ketogenic diet also targets other risk factors of periodontitis such as T2DM (by improving glycaemic control) and obesity (by reducing body fat and BMI). Overall, ketogenic diets decrease systemic inflammation and oxidative stress which could be beneficial in reducing periodontal inflammation and promoting periodontal health.
However, the potential of the ketogenic diet is still not well established in scientific literature as molecular mechanisms are poorly understood. More specifically, research regarding the diet’s usefulness in mitigating periodontal inflammation is still in its infancy. This opens a new and exciting research direction in the field of periodontal therapy. Since scientific evidence is scarce regarding the therapeutic potential of the ketogenic diet against periodontitis, future studies need to evaluate the effectiveness and safety of the diet in preventing and treating periodontitis.
Data availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gropper SS. The role of nutrition in chronic disease. Nutrients. 2023;15(3):664. https://doi.org/10.3390/NU15030664.
Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185–98. https://doi.org/10.1016/J.MCNA.2016.06.003.
Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–53. https://doi.org/10.1177/0022034514552491.
Richards D, Marcenes W, Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, et al. Review finds that severe periodontitis affects 11% of the world population. Evid Based Dent. 2014;15:70–1. https://doi.org/10.1038/SJ.EBD.6401037.
Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J Clin Periodontol. 2021;48:1165–88. https://doi.org/10.1111/JCPE.13506.
Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol. 2000;2013(62):59–94. https://doi.org/10.1111/J.1600-0757.2012.00457.X.
Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. The role of nutrition in periodontal health: An update. Nutrients. 2016;8:530. https://doi.org/10.3390/NU8090530.
Casarin M, da Silveira TM, Bezerra B, Pirih FQ, Pola NM. Association between different dietary patterns and eating disorders and periodontal diseases. Front Oral Health. 2023;4:1152031. https://doi.org/10.3389/FROH.2023.1152031/BIBTEX.
•• Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Sig Transduct Target Ther. 2022;7(1):1–21. https://doi.org/10.1038/s41392-021-00831-w. (A comprehensive review of ketogenic diets and their potential to prevent and treat various diseases.)
Murphy MM, Barraj LM, Higgins KA. Healthy U.S.-style dietary patterns can be modified to provide increased energy from protein. Nutr J. 2022;21:1–15. https://doi.org/10.1186/S12937-022-00794-W/TABLES/5.
Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48(3):e218–e218. https://doi.org/10.1038/emm.2015.122.
•• Kolb H, Kempf K, Röhling M, Lenzen-Schulte M, Schloot NC, Martin S. Ketone bodies: from enemy to friend and guardian angel. BMC Med. 2021;19(1):1–15. https://doi.org/10.1186/S12916-021-02185-0. (This review explains the various cellular mechanisms that are altered during ketogenic diets.)
Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16(1):1–10. https://doi.org/10.1186/S12916-018-1225-1.
Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262. https://doi.org/10.1016/J.CMET.2016.12.022.
McPherson PAC, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem. 2012;68:141–51. https://doi.org/10.1007/S13105-011-0112-4.
Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF. Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–7. https://doi.org/10.1080/152165401753311780.
Achanta LB, Rae CD. β-Hydroxybutyrate in the brain: One molecule, multiple mechanisms. Neurochem Res. 2017;42:35–49. https://doi.org/10.1007/S11064-016-2099-2.
Owen OE. Ketone bodies as a fuel for the brain during starvation. BAMBED. 2005;33:246–51. https://doi.org/10.1002/BMB.2005.49403304246.
Elamin M, Ruskin DN, Masino SA, Sacchetti P. Ketone-Based Metabolic Therapy: Is Increased NAD+ a Primary Mechanism? Front Mol Neurosci. 2017;10:377. https://doi.org/10.3389/FNMOL.2017.00377.
Poff AM, Moss S, Soliven M, D’Agostino DP. Ketone supplementation: Meeting the needs of the brain in an energy crisis. Front Nutr. 2021;8:783659. https://doi.org/10.3389/fnut.2021.783659.
Kumar PS. Microbial dysbiosis: The root cause of periodontal disease. J Periodontol. 2021;92:1079–87. https://doi.org/10.1002/JPER.21-0245.
Isola G, Polizzi A, Santonocito S, Alibrandi A, Williams RC. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J Periodontol. 2022;93:135–45. https://doi.org/10.1002/JPER.21-0049.
Zhao Y, Quan Y, Lei T, Fan L, Ge X, Hu S. The role of inflammasome NLPR3 in the development and therapy of periodontitis. Int J Med Sci. 2022;19:1603–14. https://doi.org/10.7150/IJMS.74575.
Ambili R, Santhi WS, Janam P, Nandakumar K, Pillai MR. Expression of activated transcription factor nuclear factor-κB in periodontally diseased tissues. J Periodontol. 2005;76:1148–53. https://doi.org/10.1902/JOP.2005.76.7.1148.
Arabaci T, Cicek Y, Canakci V, Fatih Canakci C, Ozgoz M, Albayrak M, et al. Immunohistochemical and stereologic analysis of NF-κB activation in chronic periodontitis. Eur J Dent. 2010;4:454.
Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11(3):1–13. https://doi.org/10.1038/s41368-019-0064-z.
Shang J, Liu H, Zheng Y, Zhang Z. Role of oxidative stress in the relationship between periodontitis and systemic diseases. Front Physiol. 2023;14:1210449. https://doi.org/10.3389/FPHYS.2023.1210449/BIBTEX.
Paredes-Sánchez E, Montiel-Company JM, Iranzo-Cortés JE, Almerich-Torres T, Bellot-Arcís C, Almerich-Silla JM. Meta-analysis of the Use of 8-OHdG in saliva as a marker of periodontal disease. Dis Markers. 2018;2018:7916578. https://doi.org/10.1155/2018/7916578.
Mohideen K, Chandrasekaran K, Veeraraghavan H, Faizee SH, Dhungel S, Ghosh S. Meta-analysis of assessment of total oxidative stress and total antioxidant capacity in patients with periodontitis. Dis Markers. 2023;2023:1–17. https://doi.org/10.1155/2023/9949047.
Ganesan SM, Vazana S, Stuhr S. Waistline to the gumline: Relationship between obesity and periodontal disease-biological and management considerations. Periodontol. 2000;2021(87):299–314. https://doi.org/10.1111/PRD.12390.
Li Q, Wang Q, Xu W, Ma Y, Wang Q, Eatman D, et al. C-reactive protein causes adult-onset obesity through chronic inflammatory mechanism. Front Cell Dev Biol. 2020;8:18. https://doi.org/10.3389/FCELL.2020.00018/FULL.
Machado V, Botelho J, Escalda C, Hussain SB, Luthra S, Mascarenhas P, et al. Serum C-reactive protein and periodontitis: A systematic review and meta-analysis. Front Immunol. 2021;12:706432. https://doi.org/10.3389/FIMMU.2021.706432/BIBTEX.
Martinez-Herrera M, Silvestre-Rangil J, Silvestre FJ. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med Oral Patol Oral Cir Bucal. 2017;22:e708. https://doi.org/10.4317/MEDORAL.21786.
Kim CM, Lee S, Hwang W, Son E, Kim TW, Kim K, et al. Obesity and periodontitis: A systematic review and updated meta-analysis. Front Endocrinol. 2022;13:999455. https://doi.org/10.3389/FENDO.2022.999455/BIBTEX.
de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97. https://doi.org/10.1016/J.FEBSLET.2007.11.057.
Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23(1):1–18. https://doi.org/10.1186/S12929-016-0303-Y.
Borgnakke WS. Current scientific evidence for why periodontitis should be included in diabetes management. Front Clin Diabetes Healthc. 2024;4:1257087. https://doi.org/10.3389/FCDHC.2023.1257087.
Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol. 2000;2020(82):214–24. https://doi.org/10.1111/PRD.12318.
Polak D, Sanui T, Nishimura F, Shapira L. Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontol. 2000;2020(83):46–58. https://doi.org/10.1111/PRD.12298.
Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:1–15. https://doi.org/10.1186/S12903-020-01180-W/FIGURES/4.
Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, et al. Periodontal infection, systemic inflammation, and insulin resistanceresults from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2012;35:2235–42. https://doi.org/10.2337/DC12-0072.
• Santonocito S, Giudice A, Polizzi A, Troiano G, Merlo EM, Sclafani R, Grosso G, Isola G. A cross-talk between diet and the oral microbiome: balance of nutrition on inflammation and immune system’s response during periodontitis. Nutrients. 2022;14(12):2426. https://doi.org/10.3390/NU14122426. (This review explores the importance of diet in the pathogenesis of periodontal inflammation.)
Karimi E, Yarizadeh H, Setayesh L, Sajjadi SF, Ghodoosi N, Khorraminezhad L, Mirzaei K. High carbohydrate intakes may predict more inflammatory status than high fat intakes in pre-menopause women with overweight or obesity: a cross-sectional study. BMC Res Notes. 2021;14(1):279. https://doi.org/10.1186/S13104-021-05699-1.
De Almeida-Souza CB, Antunes MM, Carbonera F, Godoy G, Da Silva MARCP, Masi LN, et al. A high-fat diet induces lower systemic inflammation than a high-carbohydrate diet in mice. Metab Syndr Relat Disord. 2021;19:296–304. https://doi.org/10.1089/MET.2020.0116.
Gomes JAS, Silva JF, Marçal AP, Silva GC, Gomes GF, de Oliveira ACP, et al. High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice. J Nutr Biochem. 2020;77:108317. https://doi.org/10.1016/J.JNUTBIO.2019.108317.
Chapple ILC, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44:S39-51. https://doi.org/10.1111/JCPE.12685.
Hujoel PP, Lingström P. Nutrition, dental caries and periodontal disease: a narrative review. J Clin Periodontol. 2017;44:S79-84. https://doi.org/10.1111/JCPE.12672.
Martinon P, Fraticelli L, Giboreau A, Dussart C, Bourgeois D, Carrouel F. Nutrition as a key modifiable factor for periodontitis and main chronic diseases. J Clin Med. 2021;10:197. https://doi.org/10.3390/JCM10020197.
Millen AE, Dahhan R, Freudenheim JL, Hovey KM, Li L, McSkimming DI, et al. Dietary carbohydrate intake is associated with the subgingival plaque oral microbiome abundance and diversity in a cohort of postmenopausal women. Sci Rep. 2022;12:2643. https://doi.org/10.1038/S41598-022-06421-2.
Monson KR, Peters BA, Usyk M, Um CY, Oberstein PE, McCullough ML, et al. Elevated dietary carbohydrate and glycemic intake associate with an altered oral microbial ecosystem in two large U.S. cohorts. CRC. 2022;2:1558–68. https://doi.org/10.1158/2767-9764.CRC-22-0323.
Angarita-Díaz MdelP, Fong C, Bedoya-Correa CM, Cabrera-Arango CL. Does high sugar intake really alter the oral microbiota?: A systematic review. Clin Exp Dent Res. 2022;8:1376–90. https://doi.org/10.1002/CRE2.640.
Li P, Li L, Zhang C, Cheng X, Zhang Y, Guo Y, et al. Palmitic acid and β-hydroxybutyrate induce inflammatory responses in bovine endometrial cells by activating oxidative stress-mediated NF-κB signaling. Molecules. 2019;24:2421. https://doi.org/10.3390/MOLECULES24132421.
Shen T, Li X, Loor JJ, Zhu Y, Du X, Wang X, et al. Hepatic nuclear factor kappa B signaling pathway and NLR family pyrin domain containing 3 inflammasome is over-activated in ketotic dairy cows. J Dairy Sci. 2019;102:10554–63. https://doi.org/10.3168/jds.2019-16706.
Shi X, Li X, Li D, Li Y, Song Y, Deng Q, et al. β-Hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol Biochem. 2014;33:920–32. https://doi.org/10.1159/000358664.
Shi X, Li D, Deng Q, Peng Z, Zhao C, Li X, et al. Acetoacetic acid induces oxidative stress to inhibit the assembly of very low density lipoprotein in bovine hepatocytes. J Dairy Res. 2016;83:442–6. https://doi.org/10.1017/S0022029916000546.
Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: Potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:5157645. https://doi.org/10.1155/2018/5157645.
Agathokleous E, Calabrese EJ. Hormesis: A general biological principle. Chem Res Toxicol. 2022;35:547–9. https://doi.org/10.1021/ACS.CHEMRESTOX.2C00032/ASSET/IMAGES/MEDIUM/TX2C00032_0004.GIF.
Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017;69:305–14. https://doi.org/10.1002/IUB.1627.
Han YM, Ramprasath T, Zou MH. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med. 2020;52:548–55. https://doi.org/10.1038/S12276-020-0415-Z.
Wang L, Chen P, Xiao W. β-hydroxybutyrate as an anti-aging metabolite. Nutrients. 2021;13(10):3420. https://doi.org/10.3390/nu13103420.
Vomhof-DeKrey EE, Picklo MJ. The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 2012;23:1201–6. https://doi.org/10.1016/J.JNUTBIO.2012.03.005.
Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–39. https://doi.org/10.1055/S-0028-1088302.
Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238–44. https://doi.org/10.1016/J.NBD.2010.05.030.
Lu Y, Yang YY, Zhou MW, Liu N, Xing HY, Liu XX, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13–8. https://doi.org/10.1016/J.NEULET.2018.06.016.
Greco T, Glenn TC, Hovda DA, Prins ML. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. JCBFM. 2016;36:1603. https://doi.org/10.1177/0271678X15610584.
Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1(8):634–50. https://doi.org/10.1038/s43587-021-00098-4.
Tozzi R, Cipriani F, Masi D, Basciani S, Watanabe M, Lubrano C, Gnessi L, Mariani S. Ketone Bodies and SIRT1, Synergic Epigenetic Regulators for Metabolic Health: A Narrative Review. Nutrients. 2022;14(15):3145. https://doi.org/10.3390/NU14153145.
Tozzi R, Campolo F, Baldini E, Venneri MA, Lubrano C, Ulisse S, Gnessi L, Mariani S. Ketogenic Diet Increases Serum and White Adipose Tissue SIRT1 Expression in Mice. Int J Mol Sci. 2022;23(24):15860. https://doi.org/10.3390/IJMS232415860.
Yin J, Han P, Tang Z, Liu Q, Shi J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. JCBFM. 2015;35:1783. https://doi.org/10.1038/JCBFM.2015.123.
Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. WJG. 2010;16:3731. https://doi.org/10.3748/WJG.V16.I30.3731.
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7:66444–54. https://doi.org/10.18632/ONCOTARGET.12119.
Guo Q, Liu S, Wang S, Wu M, Li Z, Wang Y. Beta-hydroxybutyric acid attenuates neuronal damage in epileptic mice. Acta Histochem. 2019;121:455–9. https://doi.org/10.1016/J.ACTHIS.2019.03.009.
Rahman MS, Hossain KS, Das S, Kundu S, Adegoke EO, Rahman MA, Hannan MA, Uddin MJ, Pang MG. Role of Insulin in Health and Disease: An Update. Int J Mol Sci. 2021;22(12):6403. https://doi.org/10.3390/IJMS22126403.
Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: A systematic review and meta-analysis. Obes Facts. 2021;14:222–45. https://doi.org/10.1159/000515381.
Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5–16. https://doi.org/10.1007/S11154-019-09514-Y.
Paoli A. Ketogenic diet for obesity: Friend or foe? Int J Environ Res Public Health. 2014;11:2092. https://doi.org/10.3390/IJERPH110202092.
Brand-Miller J, McMillan-Price J, Steinbeck K, Caterson I. Dietary glycemic index: health implications. J Am Coll Nutr. 2009;28(Suppl):446S-449S. https://doi.org/10.1080/07315724.2009.10718110.
Dommisch H, Kuzmanova D, Jönsson D, Grant M, Chapple I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol. 2000;2018(78):129–53. https://doi.org/10.1111/PRD.12233.
Li XY, Liu H, Zhang LY, Yang XT. The association of healthy eating index with periodontitis in National Health and Nutrition Examination Study 2011–2012. Front Nutr. 2022;9:999620. https://doi.org/10.3389/FNUT.2022.999620/BIBTEX.
Tennert C, Reinmuth AC, Bremer K, Al-Ahmad A, Karygianni L, Hellwig E, et al. An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. Microbiologyopen. 2020;9:e1056. https://doi.org/10.1002/MBO3.1056.
Gram-Kampmann EM, Olesen TB, Hansen CD, Hugger MB, Jensen JM, Handberg A, et al. A six-month low-carbohydrate diet high in fat does not adversely affect endothelial function or markers of low-grade inflammation in patients with type 2 diabetes: an open-label randomized controlled trial. Cardiovasc Diabetol. 2023;22:1–10. https://doi.org/10.1186/S12933-023-01956-8/TABLES/3.
Liu W, Zhang W, Ye M. Association between carbohydrate-to-fiber ratio and the risk of periodontitis. J Dent Sci. 2024;19:246–53. https://doi.org/10.1016/J.JDS.2023.04.012.
Woelber JP, Bremer K, Vach K, König D, Hellwig E, Ratka-Krüger P, et al. An oral health optimized diet can reduce gingival and periodontal inflammation in humans - a randomized controlled pilot study. BMC Oral Health. 2016;17:1–8. https://doi.org/10.1186/S12903-016-0257-1/TABLES/3.
Woelber JP, Tennert C, Ernst SF, Vach K, Ratka-Krüger P, Bertz H, Urbain P. Effects of a non-energy-restricted ketogenic diet on clinical oral parameters. An exploratory pilot trial. Nutrients. 2021;13(12):4229. https://doi.org/10.3390/NU13124229.
Taher HA, Salah A, Rammal C, Varma SR. Role of ketogenic diet and its effect on the periodontium. A scoping review. Front Oral Health. 2024;5:1364578. https://doi.org/10.3389/FROH.2024.1364578.
Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, et al. Inflammatory links between high fat diets and diseases. Front Immunol. 2018;9:2649. https://doi.org/10.3389/FIMMU.2018.02649.
Blasco-Baque V, Serino M, Vergnes JN, Riant E, Loubieres P, Arnal JF, Gourdy P, Sixou M, Burcelin R, Kemoun P. High-fat diet induces periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: protective action of estrogens. PLoS One. 2012;7(11):e48220. https://doi.org/10.1371/JOURNAL.PONE.0048220.
Fujita Y, Maki K. High-fat diet-induced obesity triggers alveolar bone loss and spontaneous periodontal disease in growing mice. BMC Obes. 2016;3:1–9. https://doi.org/10.1186/S40608-016-0082-8/FIGURES/7.
Brasil SC, Santos RMM, Fernandes A, Lima RS, Costa CAS, Pinto KMMDC, et al. Influence of a high-fat diet in the progression of apical periodontitis. J Endod. 2021;47:600–5. https://doi.org/10.1016/j.joen.2020.12.015.
Fernandez ML, Murillo AG. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients. 2022;14(10):2168. https://doi.org/10.3390/NU14102168.
Kratz M. Dietary cholesterol, atherosclerosis and coronary heart disease. Handb Exp Pharmacol. 2005;170:195–213. https://doi.org/10.1007/3-540-27661-0_6/COVER.
Soliman GA. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients. 2018;10(6):780. https://doi.org/10.3390/NU10060780.
Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2015;102:276–94. https://doi.org/10.3945/AJCN.114.100305.
Crosier R, McPherson R. Profound elevation in LDL cholesterol level following a ketogenic diet: A case series. CJC Open. 2022;4:732–4. https://doi.org/10.1016/j.cjco.2022.05.001.
Burén J, Ericsson M, Damasceno NRT, Sjödin A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: A randomized controlled feeding trial. Nutrients. 2021;13:814. https://doi.org/10.3390/NU13030814.
Schmidt T, Harmon DM, Kludtke E, Mickow A, Simha V, Kopecky S. The impact of the ketogenic diet on cholesterol levels in “Hyper Responders.” Am J Prev Cardiol. 2023;15:100548. https://doi.org/10.1016/J.AJPC.2023.100548.
Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. Lancet. 2020;396:1644–52. https://doi.org/10.1016/S0140-6736(20)32233-9.
Park CS, Yang HM, Han K, Lee HS, Kang J, Han JK, et al. J-shaped association between LDL cholesterol and cardiovascular events: A longitudinal primary prevention cohort of over 2.4 million people nationwide. J Adv Res. 2023. https://doi.org/10.1016/J.JARE.2023.05.003.
Ravnskov U, de Lorgeril M, Diamond DM, Hama R, Hamazaki T, Hammarskjöld B, et al. LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature. Expert Rev Clin Pharmacol. 2018;11:959–70. https://doi.org/10.1080/17512433.2018.1519391.
Froyen E. The effects of fat consumption on low-density lipoprotein particle size in healthy individuals: a narrative review. Lipids Health Dis. 2021;20(1):1–21. https://doi.org/10.1186/S12944-021-01501-0.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal The authors did not receive any funding for the submitted work.
Author information
Authors and Affiliations
Contributions
1. S.K contributed to the main text; supervised, and guided the writing process, helped in revising the manuscript; and gave the final approval for the manuscript.
2. S contributed to the main text.
3. R.A contributed to the main text.
4. B.M contributed to the main text.
5. S.S contributed to the main text, conceived the conceptual design and structure of the manuscript; revised the manuscript; and designed the original figures in the manuscript.
All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
No animal or human subjects were used by the authors in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karmakar, S., Shivaprasad, Arangaraju, R. et al. Ketogenic Diets Hold Therapeutic Potential Against Periodontal Inflammation. Curr Oral Health Rep (2024). https://doi.org/10.1007/s40496-024-00376-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s40496-024-00376-1