Abstract
Purpose of Review
While kidney transplantation improves the long-term survival of the majority of patients with end-stage kidney disease (ESKD), age-related immune dysfunction and associated comorbidities make older transplant recipients more susceptible to complications related to immunosuppression. In this review, we discuss appropriate management of immunosuppressive agents in older adults to minimize adverse events, avoid acute rejection, and maximize patient and graft survival.
Recent Findings
Physiological changes associated with senescence can impact drug metabolism and increase the risk of post-transplant infection and malignancy. Clinical trials assessing the safety and efficacy of immunosuppressive agents in older adults are lacking. Recent findings from U.S. transplant registry–based studies suggest that risk-adjusted death-censored graft failure is higher among older patients who received antimetabolite avoidance, mammalian target of rapamycin inhibitor (mTORi)–based, and cyclosporine-based regimens. Observational data suggest that risk-adjusted mortality may be increased in older patients who receive mTORi-based and cyclosporine-based regimens but lower in those managed with T cell induction and maintenance steroid avoidance/withdrawal.
Summary
Tailored immunosuppression management to improve patient and graft survival in older transplant recipients is an important goal of personalized medicine. Lower intensity immunosuppression, such as steroid-sparing regimens, appears beneficial whereas mTORi- and cyclosporine-based maintenance are associated with greater potential for adverse effects. Prospective clinical trials to assess the safety and efficacy of immunosuppression agents in older recipients are urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overview of Kidney Transplant Outcomes in Older Adults
Older adults (often defined as age ≥ 65 years) make up an increasing proportion of patients listed for and receiving kidney transplants worldwide [1,2,3,4,5,6,7]. In the USA, kidney transplantation for patients ≥ 65 years old increased over the past decade, from 2518 in 2008 to 4427 in 2018 [8]. This trend likely reflects the changing demographics of patients developing end-stage kidney disease (ESKD) [1,8,9,10,11], successful outcomes of kidney transplantation in older recipients, and the development of new strategies for increasing access, such as directed use of expanded criteria donor (ECD) organs.
For appropriate candidates, kidney transplantation is the best treatment for ESKD, as it results in improved survival, lower health care costs, and better quality of life than treatment with dialysis [4,12]. Although the absolute survival benefit of kidney transplant is greater in younger ESKD patients, patients of all age groups gain additional years-of-life with a kidney transplant compared with those who remain on dialysis [4,5]. The survival benefit of kidney transplantation among older adults, including those older than 75 years, has been identified in single-center and registry-based studies (Table 1) [5,6,7,13,14,15,16]. For example, in a retrospective registry study of patients age 70 years and older (1990–2004), Rao et al. found a 41% reduction in mortality after transplant compared to remaining on the waitlist [17]. A survival advantage was also observed in older patients who received ECD kidneys and in those with significant comorbidities including diabetes [17]. Recent data confirms the benefit of transplant with higher risk kidneys, as defined by higher kidney donor profile index (KDPI) for recipients older than 60 years [18]. Given these benefits, international guidelines recommend against the use of advanced age as an absolute exclusion criterion for kidney transplant [19,20]. However, some transplant programs currently offer kidney transplantation only to older candidates with living donors, due to concern for mortality while awaiting a deceased donor transplant offer [21,22].
Despite survival benefits compared to dialysis, older patients experience lower patient and graft survival than younger recipients [5,8,23,24,25]. The primary cause of allograft loss in older recipients is death with a functioning graft. Death with graft function is most commonly a result of cardiovascular disease, infection, or cancer [26,27,28]. Although older recipient age is an important risk factor for allograft failure, this is largely due to increased mortality, as death-censored survival analyses reveal comparable allograft survival among older and younger recipients [1,2,29,30]. In a series of Scottish transplant recipients, Oniscu et al. found equivalent 8-year death-censored graft survival regardless of recipient age [30]. Furthermore, two prospective studies suggest that rates of death-censored graft loss are lower in older adults due to the reduced incidence of acute rejection [31,32].
While the number of kidney transplants among older adults has been increasing, no specific recommendations have been formalized for the management of older kidney transplant recipients [33,34]. Prospective multicenter randomized controlled trials assessing immunosuppressive agents in older recipients are currently not available because older patients are often excluded from clinical trials [33,35]. Therefore, data on outcomes is generally derived from case series and retrospective registry–based analyses. This review considers management of immunosuppression for older kidney transplant recipients, with a focus on minimizing immunosuppression-related morbidity and mortality.
Immune Changes with Aging: Immunosenescence
Immunosenescence encompasses a series of aging-induced modifications in the immune system which are primarily characterized by dysfunctional immune responses and increased systemic inflammation termed as inflamm-aging [36,37,38,39,40,41]. Immunosenescence affects all immune compartments, with the most striking changes seen in the phenotypes and functions of CD4+ and CD8+ T cell components, and less frequently observed in components of the innate immune system [42,43,44]. Thymic involution plays a crucial role in T cell immunosenescence [45]. Patients age 60 years and older experience reductions in circulating naïve T cells, CD4 T cell receptor (TCR) excision circles, markers of thymic output, and TCR diversity [46]. The frequency of memory/effector T cells increases with age [47]. T cells downregulate the expression of the CD28 molecule with age, and subsets of CD4+/CD28− and CD8+/CD28− T cells emerge [48]. The downregulation of CD28 expression due to chronic immune activation of human T cells is one of the signatures of replicative senescence and has been associated with impaired vaccine responses in adults [49,50].
Immunosenescence leads to alteration in cellular immune function. Recently, Schaenman et al. assessed the T cell phenotype according to age by comparing 23 older (≥60 years) and 37 matched younger patients (<60 years) in the first year after transplantation [42]. The investigators demonstrated a decrease in the frequency of naïve CD4+ and CD8+ T cells among older transplant recipients compared with the younger patients. In addition, older recipients demonstrated an increase in the frequency of terminally differentiated and senescent CD8+ T cells [42]. Among older patients with infection after transplantation, there was a significantly increased frequency of T cell immune senescence [42].
Antibody responses are also decreased with age in both mice and humans, leading to increased frequency and severity of infectious diseases and reduced protective effects of vaccination [51]. Not only does the production of high-affinity protective antibodies decrease with older age, the duration of protective immunity following immunization is also shortened [51]. The decreased ability of older individuals to produce high-affinity protective antibodies against infectious agents likely results from combined defects in T cells, B cells, and other immune cells. These changes in the adaptive immune system in older patients with immunosenescence contribute to impaired ability to respond to infection, vaccination, and tumor cells [42].
Immune reconstitution after lymphocyte-depleting treatments also differs with age. Lymphocyte-depleting agents, particularly rabbit anti-thymocyte globulin (rATG), carry the risk of impaired CD4+ T cell reconstitution after induction immunosuppresion [52,53]. Previous studies showed that this risk is age-dependent and older age causes a decline in the capacity of the adult immune system to regenerate CD4+ T cells after rATG [53,54]. In a study by Longuet et al., recipient age greater than 40 years and a low CD4+ T cell count at the time of transplantation were identified as risk factors for impaired CD4+ T cell reconstitution [52].
Older kidney transplant recipients also have a higher risk of post-transplant malignancies [55,56]. A single-center analysis of 1500 kidney transplant recipients found recipient age to be an independent predictor of post-transplant malignancies [57]. The investigators demonstrated a fivefold increase in the risk of malignancy among recipients ≥60 years compared to recipients <45 years [57]. Compared to recipients 18–34 years old, an analysis of United States Renal Data System (USRDS) and Medicare billing claims data demonstrated a threefold increase in the risk of cancers among recipients 50–64 years and a fivefold increase among recipients ≥65 years [58]. Thus, there is a concern that age-related immune dysfunction can increase the susceptibility of older adults to cancer [55]. While the risk of post-transplant malignancies has been associated with the use of induction therapy with T cell depleting agents [1, 59], a recent study using USRDS and Medicare billing claims data found that the use of rATG was associated with increased post-transplant malignancy risk only among younger recipients [60•], emphasizing an important perspective that the risk of post-transplant malignancies among older recipients was not explained only by induction therapy with T cell depleting agents.
Inflamm-aging also results in chronic, low-grade, systemic inflammation characterized by a shift to the production of pro-inflammatory cytokines including IL-6, IL-1β, TNF-α, and IFN-γ, and reduction of the chemokine receptor expression and expression of several adhesion molecules [61,62]. High levels of age-associated pro-inflammatory markers are detected in the majority of older individuals, even in the absence of clinically active diseases [63,64,65]. This inflammatory status contributes to metabolic dysfunction and insulin resistance, and represents a significant risk factor for morbidity and mortality. The pro-inflammatory state has been implicated in the pathogenesis of several debilitating chronic diseases of older age including type 2 diabetes mellitus, osteoporosis, Alzheimer’s disease, rheumatoid arthritis, and coronary heart disease. In older adults, malnutrition is also common and adversely affect T cell function contributing to a state of relative immunodeficiency [66].
Immunosenescence interferes with T cell function and differentiation, assessed by flow cytometry and T cell receptors. The resulting alterations in T cell phenotype modify both rejection and tolerance [67]. Future studies are required to assess the impacts of immunosenescence and inflamm-aging in older kidney transplant recipients on tolerance induction, rejection, infection, and malignancy. In addition, further work is needed to develop methods to optimally measure the levels of immune dysfunction in older transplant recipients to successfully prevent rejection without significantly increasing the risk of infection [68]. The ability to assess T cell maturation, immune senescence, and inflamm-aging by peripheral blood mononuclear cell flow cytometry in older kidney transplant recipients may offer the potential for risk stratification and individualization of immunosuppressive therapy to optimally balance risks of rejection and infection.
The reduced risk of acute cellular rejection is consistent with thymic involution and the limited T cell receptor repertoire observed with aging [69,70,71]. Additionally, humoral immune responses in older patients are altered, with increased memory responses and a skewed B cell repertoire which is more specialized to mount humoral immune responses [72,73,74]. Together with the reduced frequency of naïve T cells, these changes are associated with impaired host defense against tumors and infections, as well as with imparied vaccine responses [72,74,75,76]. In contrast, the heightened subclinical inflammation associated with inflamm-aging and increased reactivity of the innate immune system potentiates cardiovascular risk among older transplant recipients.
Most studies comparing older with younger transplant recipients have focused on T cell responses and have described reduced frequency of acute T cell–mediated rejections in older patients [70,72,77]. However, in the few studies that investigated antibody responses, a gradual decrease in incidence of donor-specific antibodies (DSA) has been correlated with increasing chronological age [78,79]. Older kidney transplant recipients have a lower risk of developing de novo DSA than pediatric recipients, demonstrating reduced humoral immune reactivity with increasing age [80]. Increasing fundamental knowledge of how aging is involved in the immune response to organ transplantation will inform age-tailored management strategies to improve health outcomes for older transplant recipients.
Age and the Pharmacology of Immunosuppressive Drugs
Aging is associated with altered drug pharmacokinetics, including absorption, distribution across body compartments, metabolism, and excretion [81••, 82,83,84,85]. After intestinal absorption, some drugs are transported back to the intestines via P-glycoprotein (P-gp), a cell transmembrane protein with reduced expression and activity with aging, resulting in alterations of peak medication plasma concentrations and bioavailability [82]. Furthermore, bioavailability can be influenced by decreased intestinal or hepatic first-pass metabolism with aging [86]. In addition, aging is associated with an increase in relative fat content of the body and a decrease in muscle mass [82], resulting in a larger volume of distribution of lipophilic drugs such as calcineurin inhibitors (CNIs) and mammalian target of rapamycin inhibitors (mTORis) [1,55,87].
Protein production declines with aging and protein binding is decreased by up to 15 to 25% in older compared to younger adults [88]. Reduction in protein binding increases free drug concentrations. Furthermore, there is a decrease in albumin, which binds acidic drugs, and an increase in alpha-1-acid glycoprotein (AGP), which binds basic drugs [89,90]. Tacrolimus (99%), sirolimus (91%), and mycophenolic acid (MPA) (up to 97%) are highly albumin-bound compounds [82]. Protein binding is especially important in the case of MPA, in which the free fraction is the active inhibitor of inosine monophosphate dehydrogenase. Hypoalbuminemia may lead to higher pharmacologic exposure to immunosuppressive medications, especially MPA [91].
Aging is also associated with reduced renal and hepatic clearance of pharmaceuticals. The reduced renal clearance of medications has been well described with aging [92,93,94]. Drug clearance via the hepatic cytochrome P450 (CYP450) enzyme decreases with age, resulting in higher plasma levels of CNIs, mTORis, and corticosteroids [95] [96,97,98]. Older kidney transplant recipients also frequently require polypharmacy to treat comorbid conditions, and these additional medications may incur drug-drug interactions with immunosuppressive agents (Table 2) [1,99].
Calcineurin Inhibitors
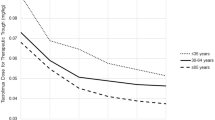
In a recent study evaluating the optimal dosing of CNIs in kidney transplant recipients >65 years, Jacobson et al. demonstrated that the normalized CNI trough concentrations were 50% greater among older recipients independent of the choice of CNI [100]. The investigators concluded that older recipients may require lower doses of CNIs to obtain the same therapeutic levels due to a decrease in metabolism from CYP3A4 isozymes and reduced P-gp activity, leading to enhanced bioavailability [81••,100]. David-Neto et al. assessed tacrolimus pharmacokinetics in 44 older kidney transplant recipients compared with 31 younger recipients [101]. Despite comparable tacrolimus trough concentrations, the older recipients had vastly different pharmacokinetics including higher observed maximum concentration (Cmax) and area under the curve (AUC), a longer time to achieve the maximum concentration, and a decreased total body clearance. Consequently, a lower total dose of tacrolimus is needed to achieve comparable immunosuppressive effects in older patients. In a study of cyclosporine pharmacokinetics, the required daily dose of cyclosporine to maintain comparable target cyclosporine concentrations was significantly lower among kidney transplant recipients age 65 years and older compared to younger recipients [102]. In addition, cyclosporine clearance was decreased, and intracellular concentrations of cyclosporine in T lymphocytes were higher in older patients [102,103].
With regard to side effects, a study of older (age ≥ 55 years) kidney transplant recipient using USRDS data (1999–2011) found associations of CNI-free maintenance immunosuppression regimen with decreased risk of dementia (HR, 0.83; P < 0.05), suggesting possible cognitive benefit of avoiding neurotoxic immunosuppression in recipients of this age group [104].
Mycophenolate
Despite receiving similar doses of MPA, Romano et al. demonstrated lower overall MPA exposure and trough concentrations when comparing 44 older (63 ± 1 years) versus 31 younger (41 ± 5 years) kidney transplant recipients [105]. Given MPA is strongly bound to serum albumin, data from liver and kidney transplant recipients showed that there was a significant higher MPA dose requirement in patients with low serum albumin levels (<3.5 g/dL) compared with recipients who had normal serum albumin [106,107]. Hypoalbuminemia can result in higher clearance of unbound MPA. As a result, older recipients commonly require higher doses of MPA compared to younger patients to achieve the same trough concentrations.
mTOR Inhibitors
Many studies evaluating mTORi pharmacokinetics included subgroup analyses of older patients and found no significant difference of drug clearance across age groups [82,108,109,110,111]. A recent study assessed the pharmacokinetics of everolimus in 16 older kidney transplant recipients receiving everolimus with low-dose tacrolimus and corticosteroids [112]. The investigators demonstrated that older patients had stable everolimus pharmacokinetic parameters without significant changes in dose or exposure during the first 6 months after kidney transplantation.
Corticosteroids
Corticosteroids are bound to albumin and corticosteroid-binding globulin (CBG). The distribution characteristics of corticosteroids are dose-dependent and nonlinear plasma protein binding. Prednisolone’s protein binding capability is decreased from 95 to 70% when higher doses are given [113]. The clearance of corticosteroids decreases with aging, resulting in enhanced exposure; however, the clinical impact of this finding requires further study [114,115].
Approach to Immunosuppression in Older Adults
Older transplant recipients comprise of a heterogeneous group and their response to immunosuppression may vary widely depending on many factors including genetic predisposition. Many biological factors such as sex, race, genetics, and other comorbidities also contribute to how older adults respond to immunosuppression regimens. Even if we define the “elderly” strictly by chronological age, younger and older individuals are likely to respond to immunosuppression differently. Biological age is likely a better predictor of how older recipients are likely to fare after transplant with certain immunosuppression regimens including immunosuppression efficacy and side effects. One of the tools that we use to determine biological age is frailty testing, such as Fried’s frailty phenotype and Karnofsky Performance Score [116••]. Some laboratory tests now are able to estimate biological age and resulting immunosenescence which may assist in profiling older recipients. Unfortunately, large-scale studies that utilize frailty or laboratory tests to determine biological age as a tool to guide immunosuppression have not been performed, but are needed to assess the benefit of these tools.
Similarly, there are no large-scale, prospective randomized clinical trials performed specifically in older transplant recipients. In fact, the majority of immunosuppression trials exclude older patients or include only a small proportion of older participants, limiting generalizability. For example, there have been two pivotal, randomized clinical trials that compared rATG to basiliximab which led to U.S. Food and Drug Administration (FDA) approval for induction indication for rATG [59]. In these two studies, less than 10% of the participants were older than 65 years. Gill et al. [10] reported a retrospective study of induction immunosuppression in 14,820 older adults in the USA. The population was classified into 4 groups based on recipient and donor risk factors: (1) high-immunologic-risk recipients/high-risk donor, (2) high-immunologic-risk recipients/low-risk donor, (3) low-immunologic-risk recipients/high-risk donor, and (4) low-immunologic-risk recipients/low-risk donor. The authors demonstrated the use of IL2-receptor antibody (IL2rAb) was associated with an increased risk for acute rejection compared with rATG in the first 3 groups (HR 1.78, 95% CI 1.34–2.35; HR 1.45, 95% CI 1.12–1.89, and HR 1.78, 95% CI 1.42–2.23), respectively. However, there was no difference in the risk of functional graft loss between the induction immunosuppression regimens. The same was observed in studies not specific to older adults; there was no significant difference in risk of acute rejection between IL2rAb and ATG in low-risk recipients/low-risk donors.
Based on the current data, the outcomes of induction immunosuppression in older adults are the same as in other populations: rATG decreases the risk of rejection and delayed graft function (DGF) and minimizes maintenance immunosuppression use without increasing complications. An approach to the choice of induction immunosuppression should consider both recipient and donor risk factors, as summarized in Tables 3 and 4. A tailored dose reduction of rATG induction in low-risk, non-sensitized recipients showed comparable outcomes of graft survival and rejection rate with typical dose recommendations of 1.5 mg/kg for up to 7 days [117]. This provides benefit among older recipients while reducing the complications.
To help address knowledge gaps using observational data, we examined associations of kidney transplant immunosuppression regimens with patient and graft survival in a retrospective cohort of older (≥ 65 years; n = 14,887) and younger (18–64 years; n = 51,475) adults using U.S. national transplant registry data (2005–2016) [118••]. We found that older transplant recipients were less likely to receive T cell depleting induction (rATG or alemtuzumab (ALEM)) with triple maintenance immunosuppression, rATG/ALEM + steroid avoidance, and mTORi-based treatment. However, older patients were more likely to receive IL2rAb + triple maintenance, IL2rAb + steroid avoidance, and cyclosporine-based regimens. Compared to older recipients treated with rATG/ALEM + triple maintenance, those who received rATG/ALEM + steroid avoidance and IL2rAb + steroid avoidance had lower risk of acute rejection, while cyclosporine-based immunosuppression was associated with borderline increased risk of acute rejection (Fig. 1). Compared to those who were treated with rATG/ALEM + triple maintenance, older recipients treated with Tac + antimetabolite avoidance, mTORi-based, and cyclosporine-based regimens had significantly (1.78-fold, 2.14-fold, and 1.78-fold, respectively) increased risks of death-censored graft failure. Further, we found that mTORi-based and cyclosporine-based regimens were associated with increased mortality (Fig. 1). Thus, these findings suggest that lower intensity immunosuppression regimens such as steroid-sparing may be beneficial for older kidney transplant recipients. Conversely, the use of mTORi and cyclosporine-based maintenance immunosuppression among older recipients should be discouraged or used cautiously due to higher risk of adverse outcomes.
Relative risks of a acute rejection, b death-censored graft failure, c death, and d all-cause graft failure according to early immunosuppression regimen and recipient age. Confidence intervals designate comparison of each regimen to the reference regimen, within age groups. *P < 0.05 for test of interaction of age group and regimen effects. Reproduced with permission from Lentine et al. [118••] and Wolters Kluwer
Future Investigations
To strengthen the evidence for tailored immunosuppression choice, ongoing research needs to define the balance of adequate immunosuppression, determined by the absence of rejection, with the risk of complications. In general, older recipients appear to have a lower risk of cellular rejection than younger patients and may require less intense immunosuppression. However, it is important to note that the consequences of rejection are likely to be more severe in older recipients. Furthermore, older recipients are more likely to receive higher KPDI kidneys which are at risk for DGF, which in turn increases the risk of rejection. Acute cellular rejection could lead to more severe and permanent damage in already compromised renal allografts. Older patients are also more likely to have adverse effects from maintenance immunosuppression and rejection treatments, including infection, cancer, post-transplant diabetes, and CNI-related nephrotoxicity. As such, recipient comorbidity, immunologic risk profile, and donor quality factors should all be taken into account when individualizing immunosuppression regimen. The initial immunosuppression plan should assess the need for and choice of induction agent, what combination of maintenance immunosuppression regimen will be used, and whether minimization of certain maintenance immunosuppression can be considered. During the course of transplant, maintenance immunosuppression regimen may need to be further adjusted when efficacy or side effects arise.
Based on these considerations, we can stratify older recipients according to lower versus higher numbers of comorbidities; classify immunologic risk as low versus high; and grade the donor quality as optimal versus less than optimal (Table 4). Recipients with a higher number of comorbidities, who will be receiving a living donor allograft, have no sensitizing events and have no DSA will be good candidates for a less intense maintenance immunosuppression. In contrast, older recipients with no comorbidities who are receiving a suboptimal deceased donor transplant and have DSA are likely to require more intense immunosuppression, both in the form of induction and maintenance therapy.
Conclusions
Aging is associated with altered pharmacodynamics, pharmacokinetics, and immune responses. While older recipients have a lower incidence of acute rejection, they face a significantly higher risk of allograft loss if they develop rejection. Personalization of immunosuppression management among older transplant recipients may be informed by consideration of recipient factors such as comorbidity burden, measures of biologic age, immunologic risk, measures of immunosenescence, and donor quality. Despite the growing number of older kidney transplant recipients, older adults continue to be under-represented in transplant clinical trials. To strengthen the evidence base for managing the care of older transplant recipients, ongoing research is needed, including robust, risk-adjusted analyses of national datasets combined with advocacy for inclusion of older adults in future prospective studies and clinical trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84(3):285–91.
Saran R, Robinson B, Abbott K. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis: the official journal of the National Kidney Foundation. 73(3 Suppl 1):A7–8.
Arns W, Citterio F, Campistol JM. ‘Old-for-old’--new strategies for renal transplantation. Nephrol Dial Transplant. 2007;22(2):336–41.
Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30.
Johnson DW, Herzig K, Purdie D, et al. A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation. 2000;69(5):794–9.
Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19(4):945–51.
Fabrizii V, Winkelmayer WC, Klauser R, et al. Patient and graft survival in older kidney transplant recipients: does age matter? J Am Soc Nephrol. 2004;15(4):1052–60.
Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant. 2020;20(Suppl s1):20–130.
Andreoni KA, Brayman KL, Guidinger MK, Sommers CM, Sung RS. Kidney and pancreas transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1359–75.
Gill J, Sampaio M, Gill JS, et al. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6(5):1168–78.
Lam NN, Kim SJ, Knoll GA, et al. The risk of cardiovascular disease is not increasing over ttime despite aging and higher comorbidity burden of kidney transplant recipients. Transplantation. 2017;101(3):588–96.
Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18(5):1168–76.
Savoye E, Tamarelle D, Chalem Y, Rebibou JM, Tuppin P. Survival benefits of kidney transplantation with expanded criteria deceased donors in patients aged 60 years and over. Transplantation. 2007;84(12):1618–24.
Gill JS, Schaeffner E, Chadban S, et al. Quantification of the early risk of death in elderly kidney transplant recipients. Am J Transplant. 2013;13(2):427–32.
Lloveras J, Arcos E, Comas J, Crespo M. Pascual J. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years. Transplantation. 2015;99(5):991–6.
Herrero JC, Gutiérrez E, Martínez A, et al. Results of kidney transplantation in recipients over 70 years of age: experience at a single center. Transplant Proc. 2003;35(5):1675–6.
Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83(8):1069–74.
Jay CL, Washburn K, Dean PG, Helmick RA, Pugh JA, Stegall MD. Survival benefit in older patients associated with earlier transplant with high KDPI kidneys. Transplantation. 2017;101(4):867–72.
Chadban SJ, Ahn C, Axelrod DA, et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104(4S1 Suppl 1):S11–103.
Segall L, Nistor I, Pascual J, et al. Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: a literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation. 2016;100(10):e55–65.
Wu C, Shapiro R, Tan H, et al. Kidney transplantation in elderly people: the influence of recipient comorbidity and living kidney donors. J Am Geriatr Soc. 2008;56(2):231–8.
Massie AB, Luo X, Lonze BE, et al. Early changes in kidney distribution under the new allocation system. J Am Soc Nephrol. 2016;27(8):2495–501.
Roodnat JI, Zietse R, Mulder PG, et al. The vanishing importance of age in renal transplantation. Transplantation. 1999;67(4):576–80.
Meier-Kriesche HU, Ojo A, Hanson J, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69(5):885–9.
Tesi RJ, Elkhammas EA, Davies EA, Henry ML, Ferguson RM. Renal transplantation in older people. Lancet. 1994;343(8895):461–4.
Karim A, Farrugia D, Cheshire J, et al. Recipient age and risk for mortality after kidney transplantation in England. Transplantation. 2014;97(8):832–8.
Kauffman HM, McBride MA, Cors CS, Roza AM, Wynn JJ. Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation. 2007;83(4):404–10.
Faravardeh A, Eickhoff M, Jackson S, et al. Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation. 2013;96(12):1089–96.
Doyle SE, Matas AJ, Gillingham K, Rosenberg ME. Predicting clinical outcome in the elderly renal transplant recipient. Kidney Int. 2000;57(5):2144–50.
Oniscu GC, Brown H, Forsythe JL. How old is old for transplantation? Am J Transplant. 2004;4(12):2067–74.
Meier-Kriesche HU, Ojo AO, Cibrik DM, et al. Relationship of recipient age and development of chronic allograft failure. Transplantation. 2000;70(2):306–10.
Lufft V, Kliem V, Tusch G, Dannenberg B, Brunkhorst R. Renal transplantation in older adults: is graft survival affected by age?A case control study. Transplantation. 2000;69(5):790–4.
KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:S1–155
Kälble T, Lucan M, Nicita G, Sells R, Revilla FB. Wiesel M. EAU guidelines on renal transplantation. Eur Urol. 2005;47(2):156–66.
Blosser CD, Huverserian A, Bloom RD, et al. Age, exclusion criteria, and generalizability of randomized trials enrolling kidney transplant recipients. Transplantation. 2011;91(8):858–63.
Heinbokel T, Elkhal A, Liu G, Edtinger K, Tullius SG. Immunosenescence and organ transplantation. Transplant Rev (Orlando). 2013;27(3):65–75.
Agrawal A, Sridharan A, Prakash S, Agrawal H. Dendritic cells and aging: consequences for autoimmunity. Expert Rev Clin Immunol. 2012;8(1):73–80.
Larbi A, Fülöp T, Pawelec G. Immune receptor signaling, aging and autoimmunity. Adv Exp Med Biol. 2008;640:312–24.
Martins PN. Impact of donor and recipient age on allograft tolerance. Exp Clin Transplant. 2009;7(2):67–77.
Thomas DR. Age-related changes in wound healing. Drugs Aging. 2001;18(8):607–20.
Sato Y, Yanagita M. Immunology of the ageing kidney. Nat Rev Nephrol. 2019;15(10):625–40.
Schaenman JM, Rossetti M, Sidwell T, et al. Increased T cell immunosenescence and accelerated maturation phenotypes in older kidney transplant recipients. Hum Immunol. 2018;79(9):659–67.
Pera A, Campos C, López N, et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82(1):50–5.
Andrés A, Budde K, Clavien PA, et al. A randomized trial comparing renal function in older kidney transplant patients following delayed versus immediate tacrolimus administration. Transplantation. 2009;88(9):1101–8.
Hirokawa K, Makinodan T. Thymic involution: effect on T cell differentiation. J Immunol. 1975;114(6):1659–64.
Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–52.
Pawelec G. Senescence: the cost of being young and healthy? Eur Cytokine Netw. 2002;13(4):387–8.
Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T cell senescence. Immunol Rev. 2005;205:158–69.
Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75(24):12182–7.
Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–9.
Frasca D, Diaz A, Romero M, Garcia D, Blomberg BB. B cell immunosenescence. Annu Rev Cell Dev Biol. 2020;36:551–74.
Longuet H, Sautenet B, Gatault P, et al. Risk factors for impaired CD4+ T-cell reconstitution following rabbit antithymocyte globulin treatment in kidney transplantation. Transpl Int. 2014;27(3):271–9.
Klaus G, Mostert K, Reckzeh B, Mueller TF. Phenotypic changes in lymphocyte subpopulations in pediatric renal-transplant patients after T-cell depletion. Transplantation. 2003;76(12):1719–24.
Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10(9):2132–41.
Saxena R, Yu X, Giraldo M, et al. Renal transplantation in the elderly. Int Urol Nephrol. 2009;41(1):195–210.
Tessari G, Naldi L, Boschiero L, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. Am J Transplant. 2013;13(1):214–21.
Danpanich E, Kasiske BL. Risk factors for cancer in renal transplant recipients. Transplantation. 1999;68(12):1859–64.
Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905–13.
Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–77.
Wang L, Motter J, Bae S, et al. Induction immunosuppression and the risk of incident malignancies among older and younger kidney transplant recipients: a prospective cohort study. Clin Transpl. 2020;34:e14121 This recent study of U.S. registry data found that, compared with IL2-receptor antibody induction, the elevated risk of post-transplant malignancy with rATG was limited to younger adult kidney transplant recipients and did not occur among adults age 65 and older.
Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18(16):1717–20.
Mo R, Chen J, Han Y, et al. T cell chemokine receptor expression in aging. J Immunol. 2003;170(2):895–904.
Ferrucci L, Semba RD, Guralnik JM, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115(18):3810–6.
Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52(4):M201–8.
Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37–46.
Lesourd BM. Nutrition and immunity in the elderly: modification of immune responses with nutritional treatments. Am J Clin Nutr. 1997;66(2):478S–84S.
Kim C, Jin J, Weyand CM, Goronzy JJ. The transcription factor TCF1 in T cell differentiation and aging. Int J Mol Sci. 2020;21(18):6497.
Martins PN, Tullius SG, Markmann JF. Immunosenescence and immune response in organ transplantation. Int Rev Immunol. 2014;33(3):162–73.
Mueller TF. Phenotypic changes with immunosuppression in human recipients. Front Biosci. 2003;8:d1254–74.
Colvin MM, Smith CA, Tullius SG, Goldstein DR. Aging and the immune response to organ transplantation. J Clin Invest. 2017;127(7):2523–9.
Heinbokel T, Hock K, Liu G, Edtinger K, Elkhal A, Tullius SG. Impact of immunosenescence on transplant outcome. Transpl Int. 2013;26(3):242–53.
de Bourcy CF, Angel CJ, Vollmers C, Dekker CL, Davis MM, Quake SR. Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proc Natl Acad Sci U S A. 2017;114(5):1105–10.
Cancro MP, Hao Y, Scholz JL, et al. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30(7):313–8.
Hao Y, O’Neill P, Naradikian MS, Scholz JL. Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–304.
Yung RL. Changes in immune function with age. Rheum Dis Clin N Am. 2000;26(3):455–73.
Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–9.
Tullius SG, Milford E. Kidney allocation and the aging immune response. N Engl J Med. 2011;364(14):1369–70.
Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99.
Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8(6):348–57.
von Moos S, Schalk G, Mueller TF, Laube G. Age-associated decrease in de novo donor-specific antibodies in renal transplant recipients reflects changing humoral immunity. Immun Ageing. 2019;16:9.
Ivulich S, Snell G. Long-term management of elderly patients taking immunosuppressive medications. Austr J Gen Pract. 2020;49(3):100–6.
Gabardi S, Tullius SG, Krenzien F. Understanding alterations in drug handling with aging: a focus on the pharmacokinetics of maintenance immunosuppressants in the elderly. Curr Opin Organ Transplant. 2015;20(4):424–30.
Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol. 2009;29(6):621–35.
Dreyer GJ, de Fijter JW. Transplanting the elderly: mandatory age- and minimal histocompatibility matching. Front Immunol. 2020;11:359.
Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62(3):248–60.
Turnheim K. Drug dosage in the elderly. Is it rational? Drugs Aging. 1998;13(5):357–79.
Hortal L, Fernández A, Losada A, et al. Study of the cyclosporine concentration at 2 hours in stable renal transplant patients and relation to body mass index. Transplant Proc. 2001;33(7-8):3110–1.
Schmucker DL. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging. 2001;18(11):837–51.
McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56(2):163–84.
Woo J, Chan HS, Or KH, Arumanayagam M. Effect of age and disease on two drug binding proteins: albumin and alpha-1- acid glycoprotein. Clin Biochem. 1994;27(4):289–92.
Nowak I, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem. 1995;41(7):1011–7.
Zeeh J, Platt D. The aging liver: structural and functional changes and their consequences for drug treatment in old age. Gerontology. 2002;48(3):121–7.
Kaplan B, Meier-Kriesche HU, Friedman G, et al. The effect of renal insufficiency on mycophenolic acid protein binding. J Clin Pharmacol. 1999;39(7):715–20.
Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J. 2012;105(8):437–45.
Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61(3):331–9.
Warrington JS, Greenblatt DJ, von Moltke LL. Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. The J Pharmacol Exp Ther. 2004;309(2):720–9.
George J, Byth K, Farrell GC. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem Pharmacol. 1995;50(5):727–30.
Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
Kinirons MT, O’Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57(5):540–4.
Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12(12):3326–36.
David-Neto E, Romano P, Kamada Triboni AH, et al. Longitudinal pharmacokinetics of tacrolimus in elderly compared with younger recipients in the first 6 months after renal transplantation. Transplantation. 2017;101(6):1365–72.
Falck P, Asberg A, Byberg KT, et al. Reduced elimination of cyclosporine A in elderly (>65 years) kidney transplant recipients. Transplantation. 2008;86(10):1379–83.
Falck P, Asberg A, Guldseth H, et al. Declining intracellular T-lymphocyte concentration of cyclosporine a precedes acute rejection in kidney transplant recipients. Transplantation. 2008;85(2):179–84.
McAdams-DeMarco MA, Bae S, Chu N, et al. Dementia and Alzheimer’s disease among older kidney transplant recipients. J Am Soc Nephrol. 2017;28(5):1575–83.
Romano P, Agena F, Ebner P, et al. Pharmacokinetics of Mycophenolic Acid (MPA) in elderly compared to young recipients in the first year after renal transplantation. Data from the NEverOLd trial. Am JTransplant. 2015;15(suppl 3).
Tredger JM, Brown NW, Adams J, et al. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl. 2004;10(4):492–502.
van Hest RM, Mathot RA, Pescovitz MD, Gordon R, Mamelok RD, van Gelder T. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17(3):871–80.
Kovarik JM, Kahan BD, Kaplan B, et al. Longitudinal assessment of everolimus in de novo renal transplant recipients over the first post-transplant year: pharmacokinetics, exposure-response relationships, and influence on cyclosporine. Clin Pharmacol Ther. 2001;69(1):48–56.
Kovarik JM, Hsu CH, McMahon L, Berthier S, Rordorf C. Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clin Pharmacol Ther. 2001;70(3):247–54.
Kovarik JM, Eisen H, Dorent R, et al. Everolimus in de novo cardiac transplantation: pharmacokinetics, therapeutic range, and influence on cyclosporine exposure. J Heart Lung Transplant : the official publication of the International Society for Heart Transplantation. 2003;22(10):1117–25.
Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95.
David-Neto E, Agena F, Ramos F, et al. Longitudinal pharmacokinetics of everolimus when combined with low-level of tacrolimus in elderly renal transplant recipients. Transplantation. 2017;101(9):2133–8.
Bergmann TK, Barraclough KA, Lee KJ, Staatz CE. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation. Clin Pharmacokinet. 2012;51(11):711–41.
Stuck AE, Frey BM, Frey FJ. Kinetics of prednisolone and endogenous cortisol suppression in the elderly. Clin Pharmacol Ther. 1988;43(4):354–62.
Tornatore KM, Logue G, Venuto RC, Davis PJ. Cortisol pharmacodynamics after methylprednisolone administration in young and elderly males. J Clin Pharmacol. 1997;37(4):304–11.
Harhay MN, Rao MK, Woodside KJ, et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant. 2020;35(7):1099–112 This comprehensive review summarizes state-of-the-art evidence of the implications of frailty for the clinical management and outcomes of kidney transplant recipients.
Singh N, Rossi AP, Savic M, Rubocki RJ, Parker MG, Vella JP. Tailored rabbit antithymocyte globulin induction dosing for kidney transplantation. Transplant Direct. 2018;4(2):e343.
Lentine KL, Cheungpasitporn W, Xiao H, et al. Immunosuppression regimen use and outcomes in older and younger adult kidney transplant recipients: a national registry analysis. Transplantation. 2020. https://doi.org/10.1097/TP.0000000000003547This recent study of U.S. registry data provides insights into trends in immunosuppressive regimens for older adult kidney recipients, including associations with clinical outcomes. The data support initiatives to personalize the immunosuppressive regimen according to recipient and donor characteristics and limit exposure to more intense immunosuppressive regimens in older adults.
Funding
D.A., M.S., and K.L.L. are supported by grant from National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) R01DK120518. K.L.L. is supported by the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. M.K. is supported by grant number T32HS026128 from the Agency for Healthcare Research and Quality. The content, interpretation, and reporting are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the funding agencies
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Frailty and Gerontology
Wisit Cheungpasitporn and Krista L. Lentine are co-first authors.
Supplementary Information
ESM 1
(PDF 106 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheungpasitporn, W., Lentine, K.L., Tan, J.C. et al. Immunosuppression Considerations for Older Kidney Transplant Recipients. Curr Transpl Rep 8, 100–110 (2021). https://doi.org/10.1007/s40472-021-00321-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-021-00321-6