Abstract
Donation after circulatory death (DCD) has contributed significantly to kidney, lung and liver transplant activities over the last decade. With an ever increasing demand for cardiac transplantation and worsening shortages of donor hearts, there has been growing interests in transplanting hearts from DCD donors. This was initially made possible by co-locating the donor and recipient to ensure the shortest possible ischaemic time for the DCD heart. More recently, reconditioning and distant procurement of arrested DCD hearts has been achieved by using machine perfusion. Early outcomes have been very encouraging, and experience to date suggests that DCD donors can contribute significantly to the number of donor hearts available for transplantation. There are variations in the legal and ethical frameworks between countries with regard to DCD organ donation and transplant teams must work within their respective local guidelines. We review the current status of clinical DCD heart transplantation and appraise the merits of the various approaches.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34(10):1244–54.
Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am J Transplant. 2015;15(3):642–9. This review of donor heart utilization highlights the growing disparity between donor organ availability and the increasing number of patients with end stage heart failure on the heart transplant waiting list.
Stoica SC, Satchithananda DK, Charman S, Sharples L, King R, Rozario C, et al. Swan-Ganz catheter assessment of donor hearts: outcome of organs with borderline hemodynamics. J Heart Lung Transplant. 2002;21(6):615–22.
Wheeldon DR, Potter CD, Oduro A, Wallwork J, Large SR. Transforming the ‘unacceptable’ donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant. 1995;14(4):734–42.
Abuanzeh R, Hashmi F, Dimarakis I, Khasati N, Machaal A, Yonan N. Venkateswaran, “Early donor management increases the retrieval rate of hearts for transplantation in marginal donors.” Eur J Cardiothorac Surg. 2015;471(1):72–7. discussion 77.
Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty. Lancet. 2015;385(9981):2003–13.
Bando T, Date H, Minami M, Kondo T, Shiraishi T, Miyoshi S, et al. First registry report: lung transplantation in Japan: The Japanese Society of Lung and Heart-Lung Transplantation. Gen Thorac Cardiovasc Surg. 2008;56(1):17–21.
Ciria R, Briceno J, Rufian S, Luque A, Lopez-Cillero P. Donation after cardiac death: where, when, and how? Transplant Proc. 2012;44(6):1470–4.
Lawler RH, West JW, McNulty PH, Clancy EJ, Murphy RP. Homotransplantation of the kidney in the human. J Am Med Assoc. 1950;144(10):844–5.
Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76.
Hardy JD, Webb WR, Dalton ML, Walker GR. Lung homotransplantation in man. JAMA. 1963;186:1065–74.
Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41(48):1271–4.
“A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death.” JAMA. 1968;205(6):337–340.
Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27(5):2893–4.
Ridley S, Bonner S, Bray K, Falvey S, Mackay J, Manara A. Intensive Care Society’s Working Group on Organ and Tissue Donation, “UK guidance for non-heart-beating donation”. Br J Anaesth. 2005;95(5):592–5.
Snoeijs MGJ, Winkens B, Heemskerk MBA, Hoitsma AJ, Christiaans MHL, Buurman WA, et al. Kidney transplantation from donors after cardiac death: a 25-year experience. Transplantation. 2010;90(10):1106–12.
D’Alessandro AM, Hoffmann RM, Knechtle SJ, Eckhoff DE, Love RB, Kalayoglu M, et al. Controlled non-heart-beating donors: a potential source of extrarenal organs. Transplant Proc. 1995;27(1):707–9.
Steen S, Sjöberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357(9259):825–9.
Kawauchi M, Gundry SR, de Begoña JA, Razzouk AJ, Bailey LL. Utilization of pediatric donors salvaged by cardiopulmonary resuscitation. J Heart Lung Transplant. 1993;12(2):185–8.
Gundry SR, Fukushima N, Eke CC, Hill AC, Zuppan C, Bailey LL. “Successful survival of primates receiving transplantation with ‘dead,’ nonbeating donor hearts.” J Thorac Cardiovasc Surg. 1995;109(6):1097–10– discussion 1101–2.
Ali AA, White P, Xiang B, Lin H-Y, Tsui SS, Ashley E, et al. Hearts from DCD donors display acceptable biventricular function after heart transplantation in pigs. Am J Transplant. 2011;11(8):1621–32.
Ali A, White P, Dhital K, Ryan M, Tsui S, Large S. Cardiac recovery in a human non-heart-beating donor after extracorporeal perfusion: source for human heart donation? J Heart Lung Transplant. 2009;28(3):290–3.
A. O. M. R. Colleges, “A code of practice for the diagnosis and confirmation of death,” pp. 1–42, Oct. 2008.
Boucek MM, Mashburn C, Dunn SM, Frizell R, Edwards L, Pietra B, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;359(7):709–14.
D. Campbell, “Pediatric cardiac transplantation using DCD donors,” www.criticalcarecanada.compresentationspediatriccardiactransplantationusingdcddonors.pdf, 31-Oct-2014. [Online]. Available: http://www.criticalcarecanada.com/presentations/2014/pediatric_cardiac_transplantation_using_dcd_donors.pdf. [Accessed: 31-Mar-2016].
Repse S, Pepe S, Anderson J, McLean C, Rosenfeldt FL. Cardiac reanimation for donor heart transplantation after cardiocirculatory death. J Heart Lung Transplant. 2010;29(7):747–55.
Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385(9987):2577–84. This paper was instrumental in supporting the clinical use of the TransMedics OCS in clinical practice as it was proved to be non-inferior to conventional cold storage in the preservation of donor organs retrieved from DBD donors. It also highlighted the importance of further investigation into functional assessment of donor hearts which have been subject to machine perfusion.
Iyer A, Gao L, Doyle A, Rao P, Cropper JR, Soto C, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant. 2015;15(2):371–80. This study used a porcine model of orthotopic transplantation to illustrate that DCD donor hearts that underwent machine perfusion with the TransMedics OCS displayed superior viability when compared with conventional cold storage.
Dhital KK, Iyer A, Connellan M, Chew HC, Gao L, Doyle A, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385(9987):2585–91. This landmark publication of the first reported series of 3 human heart transplants carried out using DCD donors following distant procurement.
Messer S, Large S. “Resuscitating heart transplantation: the donation after circulatory determined death donor.” Eur J Cardiothorac Surg. 2015;pezv357. Description of the use of DCD donor hearts with machine perfusion 16 and functional assessment to address the significant issue of donor organ shortage in the UK.
Noterdaeme T, Detry O, Hans M-F, Nellessen E, Ledoux D, Joris J, et al. What is the potential increase in the heart graft pool by cardiac donation after circulatory death? Transpl Int. 2013;26(1):61–6.
Singhal AK, Abrams JD, Mohara J, Hasz RD, Nathan HM, Fisher CA, et al. Potential suitability for transplantation of hearts from human non-heart-beating donors: data review from the Gift of Life Donor Program. J Heart Lung Transplant. 2005;24(10):1657–64.
Acknowledgments
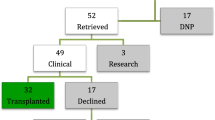
The authors are most grateful to Kumud Dhital (St. Vincent’s Hospital, Sydney, Australia) and Andre Simon (Royal Brompton and Harefield Hospital, London, UK) for sharing their data on adult DCD heart transplantation summarized in Table 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Aravinda Page and Steven Tsui declare that they have no conflicts of interest.
Stephen Ralph Large and Simon Messer report having received financial support from TransMedics towards registration and attendance at the International Society for Heart and Lung Transplantation 2016 Annual Meeting and Scientific Sessions.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Thoracic Transplantation
Rights and permissions
About this article
Cite this article
Page, A.A., Messer, S., Tsui, S.S. et al. Early Results Using Donation After Circulatory Death (DCD) Donor Hearts. Curr Transpl Rep 3, 199–206 (2016). https://doi.org/10.1007/s40472-016-0106-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-016-0106-9