Abstract
Purpose of Review
Epidemiologic evidence exists that many metals are associated with adverse neurobehavioral effects in young children, including lead (Pb), methylmercury (meHg), manganese (Mn), and arsenic (As) (Antunes dos Santos et al. J Trace Elem Med Biol. 38:99–107; Tolins et al. Ann Glob Health. 80(4):303–14; Vollet et al. Curr Environ Health Rep. 3(4):392–404; Bellinger Curr Opin Pediatr. 20(2):172–7). Importantly, chemical insult can vary depending on host factors and exposure circumstance. This systematic review summarizes the recent literature investigating modifying factors of the associations between metals and neurodevelopment, including immutable traits (sex or genetics) or exposure conditions (timing or co-exposures).
Recent Findings
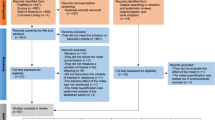
Of the 53 studies included in this review, the number investigating the modification of exposure effects was as follows: 30 for sex, 21 for co-exposures, 12 for timing of exposure, and six for genetic modifiers. Sex-specific effects of metal-neurobehavioral associations were inconclusive for all metals, likely due to the heterogeneity of outcome domains assessed and the exposure time points measured. Seven studies evaluated both sex and exposure timing as modifying factors using deciduous teeth or other biomarkers with repeated measures to characterize metals exposure over time. Only five studies used statistical methods for mixtures to evaluate associations of more than two metals with neurobehavioral domains.
Summary
Despite the expansion of research on susceptibility to the neurodevelopmental effects of metals exposure, considerable gaps remain. This work remains critical, as characterizing susceptible subpopulations can aid in identifying biological mechanisms and is fundamental for the protection of public health.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zablotsky B, Black LI, Maenner MJ, et al. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics. 2019;144(4). https://doi.org/10.1542/peds.2019-0811.
Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120(7):a258–60. https://doi.org/10.1289/ehp.1104285.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8. https://doi.org/10.1016/S1474-4422(13)70278-3.
Claus Henn B, Coull BA, Wright RO. Chemical mixtures and children’s health. Curr Opin Pediatr. 2014;26(2):223–9. https://doi.org/10.1097/MOP.0000000000000067.
Antunes dos Santos A, Appel Hort M, Culbreth M, et al. Methylmercury and brain development: a review of recent literature. J Trace Elem Med Biol. 2016;38:99–107. https://doi.org/10.1016/j.jtemb.2016.03.001.
Tolins M, Ruchirawat M, Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health. 2014;80(4):303–14. https://doi.org/10.1016/j.aogh.2014.09.005.
Vollet K, Haynes EN, Dietrich KN. Manganese exposure and cognition across the lifespan: contemporary review and argument for biphasic dose-response health effects. Curr Environ Health Rep. 2016;3(4):392–404. https://doi.org/10.1007/s40572-016-0108-x.
Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20(2):172–7. https://doi.org/10.1097/MOP.0b013e3282f4f97b.
Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. 2013;454-455:562–77. https://doi.org/10.1016/j.scitotenv.2013.03.047.
Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24(1):15–45. https://doi.org/10.1515/REVEH.2009.24.1.15.
Tsuji JS, Garry MR, Perez V, Chang ET. Low-level arsenic exposure and developmental neurotoxicity in children: a systematic review and risk assessment. Toxicology. 2015;337:91–107. https://doi.org/10.1016/j.tox.2015.09.002.
Gillies G, Virdee K, McArthur S, Neuroscience JD. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Elsevier. 2014 undefined. Accessed February 6, 2020. https://www.sciencedirect.com/science/article/pii/S0306452214004254
Bellinger DC. Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf Health Work. 2013;4(1):1–11. https://doi.org/10.5491/SHAW.2013.4.1.1.
Coetzee DJ, McGovern PM, Rao R, Harnack LJ, Georgieff MK, Stepanov I. Measuring the impact of manganese exposure on children’s neurodevelopment: advances and research gaps in biomarker-based approaches. Environ Heal A Glob Access Sci Source. 2016;15(1). https://doi.org/10.1186/s12940-016-0174-4.
Rooney JPK, Woods NF, Martin MD, Woods JS. Genetic polymorphisms of GRIN2A and GRIN2B modify the neurobehavioral effects of low-level lead exposure in children. Environ Res. 2018;165:1–10. https://doi.org/10.1016/j.envres.2018.04.001.
Andreoli V, Sprovieri F. Genetic aspects of susceptibility to mercury toxicity: an overview. Int J Environ Res Public Health. 2017;14(1). https://doi.org/10.3390/ijerph14010093.
•• Valeri L, Mazumdar MM, Bobb JF, et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect. 2017;125(6). https://doi.org/10.1289/EHP614. Associations of a mixture of Mn, Pb, and As were examined using Bayesian kernel machine regression, a statistical method for mixtures, in relation to cognition.
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res. 2016;23(9):8244–59. https://doi.org/10.1007/s11356-016-6333-x.
•• Horton MK, Hsu L, Claus Henn B, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int. 2018;121:148–58. https://doi.org/10.1016/j.envint.2018.08.045. Mn, Pb, and Zn were measured in a nearly continuous manner in tooth dentine, and associations with internalizing and externalizing behaviors were estimated with reversed distributed lag models.
•• Claus Henn B, Austin C, Coull BA, et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res. 2018;161:588–98. https://doi.org/10.1016/j.envres.2017.12.003. Modification of Mn by sex, BPb, and exposure timing were evaluated in relation to visual-spatial scores, using tooth dentine as an exposure biomarker to estimate exposure timing in a nearly continuous manner from the prenatal period through childhood.
Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000;108(January):511–533. https://doi.org/10.1289/ehp.00108s3511.
Shah-Kulkarni S, Ha M, Kim BM, et al. Neurodevelopment in early childhood affected by prenatal lead exposure and iron intake. Medicine. 2016;95(4). https://doi.org/10.1097/MD.0000000000002508.
Menezes-Filho JA, Carvalho CF, Rodrigues JLG, et al. Environmental co-exposure to lead and manganese and intellectual deficit in school-aged children. Int J Environ Res Public Health. 2018;15(11). https://doi.org/10.3390/ijerph15112418.
Frndak S, Barg G, Canfield RL, Quierolo EI, Mañay N, Kordas K. Latent subgroups of cognitive performance in lead- and manganese-exposed Uruguayan children: examining behavioral signatures. Neurotoxicology. 2019;73(April):188–98. https://doi.org/10.1016/j.neuro.2019.04.004.
Rodrigues EG, Bellinger DC, Valeri L, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Heal A Glob Access Sci Source. 2016;15(1). https://doi.org/10.1186/s12940-016-0127-y.
• Freire C, Amaya E, Gil F, et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: the Environment and Childhood (INMA) Project. Sci Total Environ. 2018;621:340–51. https://doi.org/10.1016/j.scitotenv.2017.11.273. Placenta was used as a biomarker of exposure to Mn, Pb, As, Mn, Hg, and Cd and examined in relation to cognition.
•• Levin-Schwartz Y, Gennings C, Schnaas L, et al. Time-varying associations between prenatal metal mixtures and rapid visual processing in children. Environ Health. 2019;18(1):92. https://doi.org/10.1186/s12940-019-0526-y. This study examined time-varying effects of a complex mixture of metals (As, Cd, Pb, cesium, chromium, antimony) during pregnancy in association with child neurobehavior, using the extensions of weighted quantile sum regression, a statistical method for mixtures.
Kordas K, Ardoino G, Coffman DL, et al. Patterns of exposure to multiple metals and associations with neurodevelopment of preschool children from Montevideo, Uruguay. J Environ Public Health. 2015;2015:1–9. https://doi.org/10.1155/2015/493471.
Silver MK, Li X, Liu Y, et al. Low-level prenatal lead exposure and infant sensory function. Environ Heal A Glob Access Sci Source. 2016;15(1). https://doi.org/10.1186/s12940-016-0148-6.
Desrochers-Couture M, Oulhote Y, Arbuckle TE, et al. Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ Int. 2018:1235–42. https://doi.org/10.1016/j.envint.2018.10.043.
• Taylor CM, Kordas K, Golding J, Emond AM. Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. Neurotoxicology. 2017;62:162–9. https://doi.org/10.1016/j.neuro.2017.07.003. This study measured prenatal Pb exposure and assessed sex differences in the association of Pb and child cognition at multiple points in childhood.
Fruh V, Rifas-Shiman SL, Amarasiriwardena C, et al. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology. 2019;75:105–15. https://doi.org/10.1016/j.neuro.2019.09.006.
Barg G, Daleiro M, Queirolo EI, et al. Association of low lead levels with behavioral problems and executive function deficits in schoolers from Montevideo, Uruguay. Int J Environ Res Public Health. 2018;15(12). https://doi.org/10.3390/ijerph15122735.
•• Al-Saleh I, Al-Mohawes S, Al-Rouqi R, Elkhatib R. Selenium status in lactating mothers-infants and its potential protective role against the neurotoxicity of methylmercury, lead, manganese, and DDT. Environ Res. 2019;176(March):108562. https://doi.org/10.1016/j.envres.2019.108562. Multiple biomarkers for Mn, Hg, and other metals were evaluated using principal component analysis in relation to cognitive and other behavioral outcomes.
Strain JJ, Yeates AJ, Van Wijngaarden E, et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. 2015;101(3):530–7. https://doi.org/10.3945/ajcn.114.100503.
Golding J, Hibbeln JR, Gregory SM, Iles-Caven Y, Emond A, Taylor CM. Maternal prenatal blood mercury is not adversely associated with offspring IQ at 8 years provided the mother eats fish: a British prebirth cohort study. Int J Hyg Environ Health. 2017;220(7):1161–7. https://doi.org/10.1016/j.ijheh.2017.07.004.
Hibbeln J, Gregory S, Iles-Caven Y, Taylor CM, Emond A, Golding J. Total mercury exposure in early pregnancy has no adverse association with scholastic ability of the offspring particularly if the mother eats fish. Environ Int. 2018;116:108–15. https://doi.org/10.1016/j.envint.2018.03.024.
Llop S, Ballester F, Murcia M, et al. Prenatal exposure to mercury and neuropsychological development in young children: the role of fish consumption. Int J Epidemiol. 2017;46(3):827–38. https://doi.org/10.1093/ije/dyw259.
Xu Y, Khoury JC, Sucharew H, Dietrich K, Yolton K. Low-level gestational exposure to mercury and maternal fish consumption: associations with neurobehavior in early infancy. Neurotoxicol Teratol. 2016;54:61–7. https://doi.org/10.1016/j.ntt.2016.02.002.
Jacobson JL, Muckle G, Ayotte P, Dewailly É, Jacobson SW. Relation of prenatal methylmercury exposure from environmental sources to childhood IQ. Environ Health Perspect. 2015;123(8):827–33. https://doi.org/10.1289/ehp.1408554.
Marques RC, Bernardi JVE, Abreu L, Dórea JG. Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch Environ Contam Toxicol. 2015;68(3):432–41. https://doi.org/10.1007/s00244-014-0103-x.
Jeong KS, Park H, Ha E, et al. High maternal blood mercury level is associated with low verbal IQ in children. J Korean Med Sci. 2017;32(7):1097–104. https://doi.org/10.3346/jkms.2017.32.7.1097.
Barbone F, Rosolen V, Mariuz M, et al. Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: results from the Mediterranean PHIME cohort. Int J Hyg Environ Health. 2019;222(1):9–21. https://doi.org/10.1016/j.ijheh.2018.07.011.
Kim Y, Ha EH, Park H, et al. Prenatal mercury exposure, fish intake and neurocognitive development during first three years of life: prospective cohort mothers and Children’s environmental health (MOCEH) study. Sci Total Environ. 2018;615:1192–8. https://doi.org/10.1016/j.scitotenv.2017.10.014.
Wang J, Wu W, Li H, et al. Relation of prenatal low-level mercury exposure with early child neurobehavioral development and exploration of the effects of sex and DHA on it. Environ Int. 2019;126:14–23. https://doi.org/10.1016/j.envint.2019.02.012.
Tatsuta N, Nakai K, Sakamoto M, Murata K, Satoh H. Methylmercury exposure and developmental outcomes in Tohoku study of child development at 18 months of age. Toxics. 2018;6(3):49. https://doi.org/10.3390/toxics6030049.
Engström K, Love TM, Watson GE, et al. Polymorphisms in ATP-binding cassette transporters associated with maternal methylmercury disposition and infant neurodevelopment in mother-infant pairs in the Seychelles child development study. Environ Int. 2016;94:224–9. https://doi.org/10.1016/j.envint.2016.05.027.
•• Wahlberg K, Love TM, Pineda D, et al. Maternal polymorphisms in glutathione-related genes are associated with maternal mercury concentrations and early child neurodevelopment in a population with a fish-rich diet. Environ Int. 2018;115:142–9. https://doi.org/10.1016/j.envint.2018.03.015. This article investigated genetic polymorphisms that may modify the association between multiple prenatal meHg biomarkers and Bayley Scales of Infant Development in a large prospective birth cohort.
Snoj Tratnik J, Falnoga I, Trdin A, et al. Prenatal mercury exposure, neurodevelopment and apolipoprotein E genetic polymorphism. Environ Res. 2017;152:375–85. https://doi.org/10.1016/j.envres.2016.08.035.
Julvez J, Davey Smith G, Ring S, Grandjean P. A birth cohort study on the genetic modification of the association of prenatal methylmercury with child cognitive development. Am J Epidemiol. 2019;188(10):1784–93. https://doi.org/10.1093/aje/kwz156.
•• Llop S, Tran V, Ballester F, et al. CYP3A genes and the association between prenatal methylmercury exposure and neurodevelopment. Environ Int. 2017;105:34–42. https://doi.org/10.1016/j.envint.2017.04.013. Data from three large cohorts were combined using meta analysis to investigate whether cyp3A genetic polymorphisms modified the association between prenatal Hg and Bayley Scales of Infant Development scores.
Wang B, Liu J, Liu B, Liu X, Yu X. Prenatal exposure to arsenic and neurobehavioral development of newborns in China. Environ Int. 2018;121:421–7. https://doi.org/10.1016/j.envint.2018.09.031.
•• Signes-Pastor AJ, Vioque J, Navarrete-Muñoz EM, et al. Inorganic arsenic exposure and neuropsychological development of children of 4–5 years of age living in Spain. Environ Res. 2019;174:135–42. https://doi.org/10.1016/j.envres.2019.04.028. Authors measured speciated As levels and were therefore able to exclude arsenobetaine, a non-toxic arsenical derived from fish and seafood, from the As measure. Possible differences by child sex were also evaluated.
• Desai G, Barg G, Queirolo EI, et al. A cross-sectional study of general cognitive abilities among Uruguayan school children with low-level arsenic exposure, potential effect modification by methylation capacity and dietary folate. Environ Res. 2018;164:124–31. https://doi.org/10.1016/j.envres.2018.02.021. This paper measured speciated As levels and were therefore able to exclude arsenobetaine, a non-toxic arsenical derived from fish and seafood, from their As measure. They also investigated several modifying factors, including child sex, dietary folate, and %MMA, a possible indicator of inefficient As metabolism.
Zhou T, Guo J, Zhang J, et al. Sex-specific differences in cognitive abilities associated with childhood cadmium and manganese exposures in school-age children: a prospective cohort study. Biol Trace Elem Res. 2020;193(1):89–99. https://doi.org/10.1007/s12011-019-01703-9.
Gunier RB, Arora M, Jerrett M, et al. Manganese in teeth and neurodevelopment in young Mexican-American children. Environ Res. 2015;142:688–95. https://doi.org/10.1016/j.envres.2015.09.003.
García-Chimalpopoca Z, Hernández-Bonilla D, Cortez-Lugo M, et al. Verbal memory and learning in schoolchildren exposed to manganese in Mexico. Neurotox Res. 2019;36(4):827–35. https://doi.org/10.1007/s12640-019-00037-7.
Mora AM, Arora M, Harley KG, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. https://doi.org/10.1016/j.envint.2015.07.009.
de Carvalho CF, Oulhote Y, Martorelli M, et al. Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. Neurotoxicology. 2018;69:253–9. https://doi.org/10.1016/j.neuro.2018.02.002.
Claus Henn B, Bellinger DC, Hopkins MR, et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site. Environ Health Perspect. 2017;125(6):1–9. https://doi.org/10.1289/EHP925.
Haynes EN, Sucharew H, Hilbert TJ, et al. Impact of air manganese on child neurodevelopment in East Liverpool, Ohio. Neurotoxicology. 2018;64:94–102. https://doi.org/10.1016/j.neuro.2017.09.001.
Oulhote Y, Mergler D, Barbeau B, et al. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 2014;122(12):1343–50. https://doi.org/10.1289/ehp.1307918.
Bouchard MF, Surette C, Cormier P, Foucher D. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology. 2018;64:110–7. https://doi.org/10.1016/j.neuro.2017.07.024.
• Mora AM, Córdoba L, Cano JC, et al. Prenatal mancozeb exposure, excess manganese, and neurodevelopment at 1 year of age in the infants’ environmental health (ISA) study. Environ Health Perspect. 2018;126(5):1–9. https://doi.org/10.1289/EHP1955. Multiple Mn biomarkers in early and late parts of pregnancy were used to estimate associations between prenatal exposure and Bayley Scales of Infant Development scores.
•• Joo H, Choi JH, Burm E, et al. Gender difference in the effects of lead exposure at different time windows on neurobehavioral development in 5-year-old children. Sci Total Environ. 2018;615:1086–92. https://doi.org/10.1016/j.scitotenv.2017.10.007. Multiple time windows of prenatal Pb exposure and childhood Pb exposure were measured, and the authors assessed sex differences in the association between Pb and child neurobehavior.
Rodrigues JLG, Araújo CFS, dos Santos NR, et al. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ Res. 2018;167(May):66–77. https://doi.org/10.1016/j.envres.2018.07.007.
Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J. Bisphenol a, phthalates and lead and learning and behavioral problems in Canadian children 6-11 years of age: CHMS 2007-2009. Neurotoxicology. 2016;54:89–98. https://doi.org/10.1016/j.neuro.2016.03.014.
Ji Y, Hong X, Wang G, et al. A prospective birth cohort study on early childhood lead levels and attention deficit hyperactivity disorder: new insight on sex differences. J Pediatr. 2018;199:124–131.e8. https://doi.org/10.1016/j.jpeds.2018.03.076.
Ng S, Lin CC, Jeng SF, Hwang YH, Hsieh WS, Chen PC. Mercury, APOE, and child behavior. Chemosphere. 2015;120:123–30. https://doi.org/10.1016/j.chemosphere.2014.06.003.
•• Patel NB, Xu Y, McCandless LC, et al. Very low-level prenatal mercury exposure and behaviors in children: the HOME Study. Environ Health. 2019;18(1). https://doi.org/10.1186/s12940-018-0443-5. This study measured multiple time windows of prenatal Hg exposure and assessed sex differences in the association of Hg and child neurobehavior.
Rodríguez-Barranco M, Gil F, Hernández AF, et al. Postnatal arsenic exposure and attention impairment in school children. Cortex. 2016;74:370–82. https://doi.org/10.1016/j.cortex.2014.12.018.
Ode A, Rylander L, Gustafsson P, et al. Manganese and selenium concentrations in umbilical cord serum and attention deficit hyperactivity disorder in childhood. Environ Res. 2015;137:373–81. https://doi.org/10.1016/j.envres.2015.01.001.
Menezes-Filho JA, de Carvalho-Vivas CF, Viana GFS, et al. Elevated manganese exposure and school-aged children’s behavior: a gender-stratified analysis. Neurotoxicology. 2014;45:293–300. https://doi.org/10.1016/j.neuro.2013.09.006.
Tinkov AA, Mazaletskaya AL, Ajsuvakova OP, et al. ICP-MS assessment of hair essential trace elements and minerals in Russian preschool and primary school children with attention-deficit/hyperactivity disorder (ADHD). Biol Trace Elem Res. Published online 2019. https://doi.org/10.1007/s12011-019-01947-5
Schwartz J. Low-level Lead exposure and children′s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65(1):42–55. https://doi.org/10.1006/enrs.1994.1020.
Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS. Sex-dependent effects of developmental lead exposure on the brain. Front Genet. 2018;9(MAR):89. https://doi.org/10.3389/fgene.2018.00089
Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. https://doi.org/10.3109/10408449509089885.
Clarkson TW, Magos L, Myers GJ. The toxicology of mercury-current exposures and clinical manifestations. 2003;18. Accessed September 2, 2020. www.nejm.org
NRC. Toxicological effects of methylmercury. National Academies Press; 2000. https://doi.org/10.17226/9899
Mahaffey KR, Sunderland EM, Chan HM, et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev. 2011;69(9):493–508. https://doi.org/10.1111/j.1753-4887.2011.00415.x.
Rothenberg SE, Yu X, Liu J, et al. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: a prospective cohort study. Int J Hyg Environ Health. 2016;219(8):832–42. https://doi.org/10.1016/j.ijheh.2016.07.014.
Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–8. https://doi.org/10.1016/j.envres.2010.10.009.
Council NR. Critical aspects of EPA’s IRIS assessment of inorganic arsenic. Published online 2013.
Carignan CC, Karagas MR, Punshon T, Gilbert-Diamond D, Cottingham KL. Contribution of breast milk and formula to arsenic exposure during the first year of life in a US prospective cohort. J Expo Sci Environ Epidemiol. 2016;26(5):452–7. https://doi.org/10.1038/jes.2015.69.
Balachandran RC, Mukhopadhyay S, McBride D, et al. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem. 2020;295(19):6312–29. https://doi.org/10.1074/jbc.REV119.009453.
Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Asp Med. 2005;26(4–5 SPEC. ISS):353–62. https://doi.org/10.1016/j.mam.2005.07.003.
National Academy of Science. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press; 2001. https://doi.org/10.17226/10026
Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113(2):369–77. https://doi.org/10.1016/j.pharmthera.2006.09.002.
Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27(3):315–26. https://doi.org/10.1016/j.neuro.2005.10.007.
Guilarte TR. Manganese and Parkinson’s disease: a critical review and new findings. Environ Health Perspect. 2010;118(8):1071–80. https://doi.org/10.1289/ehp.0901748.
Miranda M, Bustamante ML, Mena F, Lees A. Original footage of the Chilean miners with manganism published in neurology in 1967. Neurology. 2015;85(24):2166–9. https://doi.org/10.1212/WNL.0000000000002223.
Funding
The research described in this paper was funded in part by NIEHS grants: F31ES029010, T32ES014562, R00 ES022986, and K99 ES030400.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Environmental Epidemiology
Rights and permissions
About this article
Cite this article
Bauer, J.A., Fruh, V., Howe, C.G. et al. Associations of Metals and Neurodevelopment: a Review of Recent Evidence on Susceptibility Factors. Curr Epidemiol Rep 7, 237–262 (2020). https://doi.org/10.1007/s40471-020-00249-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-020-00249-y