Abstract
Purpose
Photodynamic therapy (PDT) is effective in reducing pathogenic microorganisms in the oral cavity and in preventing dental diseases. This study evaluated the pre-irradiation time using PDT (diode laser associated with 0.01% methylene blue) to decrease the number of microorganisms in the visible plaque in permanent teeth.
Methods
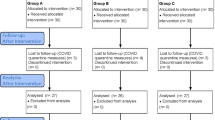
This randomized clinical trial included 108 homologous lower permanent first molars (36 and 46) with biofilm from 54 children aged six to 12 years. PDT was performed (0.01% methylene blue photosensitizer/therapeutic laser-InGaAIP), according to the following protocols: Group 1, biofilm collection of the distal area of the lingual surface of 36 µm before PDT; group 2, mesial area of the lingual surface of 36 µm 1 min after PDT; group 3, area of the lingual surface of 46 µm before PDT; and group 4, mesial area of the lingual surface of 46 µm 5 min after PDT.
Results
After statistical analysis, significant differences were observed between the groups (p = 0.000). In groups 2 and 4, the number of bacteria tended to decrease, with a more evident bacterial reduction in group 4.
Conclusions
Pre-irradiation reduced the number of colony-forming units of mature bacterial biofilms in vivo. A time of 5 min resulted in a greater reduction in the number of colony-forming units.
Clinical trial registration
ReBEC Identifier: RBR-6bqfp3; Date of Register: March 2nd, 2020. Retrospectively Registered.

Similar content being viewed by others
References
Alves LVGL, Curylofo-Zotti FA, Borsatto MC, Salvador SLS, Valério RA, Souza-Gabriel AE, Corona SAM. Influence of antimicrobial photodynamic therapy in carious lesion. Randomized split-mouth clinical trial in primary molars. Photodiagnosis Photodyn Ther. 2019;26:124–30. https://doi.org/10.1016/j.pdpdt.2019.02.018.
Azizi A, Shademan S, Rezai M, Rahimi A, Lawaf S. Effect of photodynamic therapy with two photosensitizers on Streptococcus mutants: in vitro study. Photodiagnosis Photodyn Ther. 2016;16:66–71. https://doi.org/10.1016/j.pdpdt.2016.08.002.
Belibasakis GN, Bostanci N, Marsh PD, Zaura E. Applications of the oral microbiome in personalized dentistry. Arch Oral Biol. 2019;104:7–12. https://doi.org/10.1016/j.archoralbio.2019.05.023.
Cadore UB, Reis MBL, Martins SHL, Invernici MM, Novaes AB Jr, Taba M Jr, Palioto DB, Messora MR, Souza SLS. Multiple sessions of antimicrobial photodynamic therapy associated with surgical periodontal treatment in patients with chronic periodontitis. J Periodontol. 2019;90(4):339–49. https://doi.org/10.1002/JPER.18-0373.
Carrera ET, Dias HB, Corbi SCT, Marcantonio RAC, Bernardi ACA, Bagnato VS, et al. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: a critical review. HHS Public Access. 2016;26(5):1011–4. https://doi.org/10.1088/1054-660X/26/12/123001.
Chaves Lamarque GC, Cusicanqui Méndez DA, Arruda Matos A, Dionísio TJ, Moreira Machado MA, Magalhães AC, Cardoso Oliveira R, Cruvinel T. Cytotoxic effect and apoptosis pathways activated by methylene blue-mediated photodynamic therapy in fibroblasts. Photodiagnosis Photodyn Ther. 2020. https://doi.org/10.1016/j.pdpdt.2020.101654.
Cieplik F, Tabenski L, Buchalla W, Maisch T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front Microbiol. 2014;5:405. https://doi.org/10.3389/fmicb.2014.00405.
De Sousa Farias SS, Nemezio MA, Corona SA, Aires CP, Borsatto MC. Effects of low-level laser therapy combined with toluidine blue on polysaccharides and biofilm of Streptococcus mutans. Lasers Med Sci. 2016;31(5):1011–6. https://doi.org/10.1007/s10103-016-1944-5.
Diniz IM, Horta ID, Azevedo CS, Elmadjian TR, Matos AB, Simionato MR, Marques MM. Antimicrobial photodynamic therapy: a promise candidate for caries lesions treatment. Photodiagnosis Photodyn Ther. 2015;12(3):511–8. https://doi.org/10.1016/j.pdpdt.2015.04.006.
Esteban Florez FL, Oliveira MRM, de Oliveira Júnior OB, Hiers RD, Khajotia SS, Pretel H. Bioluminescence analysis of antibacterial photodynamic therapy using methylene blue mediated by low-intensity level laser against cariogenic biofilms. Photomed Laser Surg. 2018;36(5):258–65. https://doi.org/10.1089/pho.2017.4326.
Fumes AC, da Silva Telles PD, Corona SAM, Borsatto MC. Effect of aPDT on Streptococcus mutans and Candida albicans present in the dental biofilm: systematic review. Photodiagnosis Photodyn Ther. 2018;21:363–6. https://doi.org/10.1016/j.pdpdt.2018.01.013.
Ghorbanzadeh A, Bahador A, Sarraf P, Ayar R, Fekrazad R, Asefi S. Ex vivo comparison of antibacterial efficacy of conventional chemomechanical debridement alone and in combination with light-activated disinfection and laser irradiation against enterococcus faecalis biofilm. Photodiagnosis Photodyn Ther. 2020;29: 101648. https://doi.org/10.1016/j.pdpdt.2019.101648.
Gong J, Park H, Lee J, Seo H, Lee S. Effect of photodynamic therapy on multispecies biofilms, including Streptococcus mutans, Lactobacillus casei, and Candida albicans. Photobiomodul Photomed Laser Surg. 2019;37(5):282–7. https://doi.org/10.1089/photob.2018.4571.
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y. Application of lasers in periodontics: true innovation or myth? Periodontol. 2009;50:90–126. https://doi.org/10.1111/j.1600-0757.2008.00283.x.
Larsen T, Fiehn NE. Dental biofilm infections—an update. APMIS. 2017;125(4):376–84. https://doi.org/10.1111/apm.12688.
Leal CRL, Alvarenga LH, Oliveira-Silva T, Kato IT, Godoy-Miranda B, Bussadori SK, et al. Antimicrobial photodynamic therapy on Streptococcus mutans is altered by glucose in the presence of methylene blue and red LED. Photodiagnosis Photodyn Ther. 2017;19:1–4. https://doi.org/10.1016/j.pdpdt.2017.04.004.
Legéňová K, Bujdáková H. The role of Streptococcus mutans in the oral biofilm. Epidemiol Mikrobiol Imunol. 2015;64(4):179–87.
Longo JP, Leal SC, Simioni AR, der Fátima Menezes Almeida-Santos M, Tedesco AC, Azevedo RB, Ochsner M. Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: an in vitro and clinical study. Lasers Med Sci. 2012;27:575–84. https://doi.org/10.1007/s10103-011-0962-6.
López-Gómez SA, Villalobos-Rodelo JJ, Ávila-Burgos L, Casanova-Rosado JF, Vallejos-Sánchez AA, Lucas-Rincón SE, et al. Relationship between premature loss of primary teeth with oral hygiene, consumption of soft drinks, dental care, and previous caries experience. Sci Rep. 2016;6:21147. https://doi.org/10.1038/srep21147.
Mang TS, Tayal DP, Baier R. Photodynamic therapy as an alternative treatment for disinfection of bacteria in oral biofilms. Lasers Surg Med. 2012;44:588–96. https://doi.org/10.1002/lsm.22050.
Martins SHL, Novaes AB Jr, Taba M Jr, Palioto DB, Messora MR, Reino DM, Souza SLS. Effect of surgical periodontal treatment associated to antimicrobial photodynamic therapy on chronic periodontitis: a randomized controlled clinicaltrial. J Clin Periodontol. 2017;44(7):717–28. https://doi.org/10.1111/jcpe.12744.
Meeker DG, Jenkins SV, Miller EK, Beenken KE, Loughran AJ, Powless A, Muldoon TJ, Galanzha EI, Zharov VP, Smeltzer MS, Chen J. Synergistic photothermal and antibiotic killing of biofilm-associated Staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect Dis. 2016;2(4):241–50. https://doi.org/10.1021/acsinfecdis.5b00117.
Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials. 2009;10:27. https://doi.org/10.1186/1745-6215-10-27.
Misba L, Zaidi S, Khan AU. Efficacy of photodynamic therapy against Streptococcus mutans biofilm: Role of singlet oxygen. J Photochem Photobiol B. 2018;183:16–21. https://doi.org/10.1016/j.jphotobiol.2018.04.024.
Monzavi A, Chinipardaz Z, Mousavi M, Fekrazad R, Moslemi N, Azaripour A, Bagherpasand O, Chiniforush N. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: a randomized clinical trial. Photodiagnosis Photodyn Ther. 2016;14:93–7. https://doi.org/10.1016/j.pdpdt.2016.02.007.
Nemezio MA, de Souza Farias SS, Borsatto MC, Aires CP, Corona SAM. Effect of methylene blue-induced photodynamic therapy on a Streptococcus mutans biofilm model. Photodiagnosis Photodyn Ther. 2017;20:234–7. https://doi.org/10.1016/j.pdpdt.2017.10.025.
Nour El Din S, El-Tayeb TA, Abou-Aisha K, El-Azizi M. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int J Nanomed. 2016;11:1749–58. https://doi.org/10.2147/IJN.S102398.
O’Neill JF, Hope CK, Wilson M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg Med. 2002;31:86–90. https://doi.org/10.1002/lsm.10087.
Pourhajibaghera M, Kazemian H, Chiniforush N, Hosseini N, Pourakbari B, Azizollahi A, Rezaei F, Bahador A. Exploring different photosensitizers to optimize elimination of planktonic and biofilm forms of Enterococcus faecalis from infected root canal during antimicrobial photodynamic therapy. Photodiagnosis Photodyn Ther. 2018;24:206–11. https://doi.org/10.1016/j.pdpdt.2018.09.014.
Reis ACM, Regis WFM, Rodrigues LKA. Scientific evidence in antimicrobial photodynamic therapy: an alternative approach for reducing cariogenic bacteria. Photodiagnosis Photodyn Ther. 2019;26:179–89. https://doi.org/10.1016/j.pdpdt.2019.03.012.
Saleem HGM, Seers CA, Sabri AN, Reynolds EC. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016;16:214. https://doi.org/10.1186/s12866-016-0833-1.
Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. https://doi.org/10.3109/00016356408993968.
Soares LGP, Crugeira PJL, Nunes IPF, Santos AS, Cangussú MCT, de Almeida PF, Pinheiro ALB, Habib FAL. Oral microbiological control by photodynamic action in orthodontic patients. Photodiagnosis Photodyn Ther. 2019;28:221–5. https://doi.org/10.1016/j.pdpdt.2019.08.002.
Teixeira AH, Pereira ES, Rodrigues LK, Saxena D, Duarte S, Zanin IC. Effect of photodynamic antimicrobial chemotherapy on in vitro and in situ biofilms. Caries Res. 2012;46:549–54. https://doi.org/10.1159/000341190.
Funding
The authors did not receive support from any organisation for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The patients’ selection and material preparation were done by NGL, CPT and RMM. EW and RMM analyses and process the sample samples at the Laboratory of the Center for the Study of Prevention and Control of Infection in Health Services (NEPECISS) of the School of Nursing of Ribeirão Preto-University of São Paulo (EERP-USP). The statistical analysis were performed by AES-G. The first draft of the manuscript was written by NGL, CPT, MCB and EW, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conficts of interest to declare.
Ethical approval
This study was approved by the Ethics in Research Committee (protocol CAAE-00781718.5.0000.5419). Written informed consent form was obtained from all volunteers/legal guardians. This clinical trial (CT) was registered in the Clinical Trials Registry (ReBEC) under the identifier # RBR-6bqfp3.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lima, N.G., Monteiro, R.M., Torres, C.P. et al. Influence of antimicrobial photodynamic therapy with different pre-irradiation times on children’s dental biofilm: randomized clinical trial. Eur Arch Paediatr Dent 23, 897–904 (2022). https://doi.org/10.1007/s40368-022-00716-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40368-022-00716-8