Abstract
Introduction
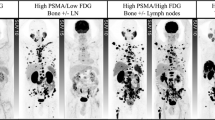
In recent years, prostate specific membrane antigen (PSMA) has been gaining a crucial role for prostate cancer (PC) management, representing an ideal platform to combine diagnosis and therapy in a unique approach, namely theranostics. However, low or absent PSMA expression has been reported in up to 20% of PC cases. Our aim was to review the applications of PET/CT with radiolabeled antibodies (immunoPET) to identify biomarkers other than PSMA, potentially suitable for PC theranostics.
Methods
We performed a Pubmed/Medline research to identify the most relevant findings of the literature published to date on this topic.
Result
Prostate stem cell antigen (PSCA), a biomarker strongly overexpressed in metastatic castration-resistant PC (mCRPC), was effectively imaged in animal models through immunoPET with 124I and 89Zr-conjugated antibody fragments (minibodies) and gave promising results as a theranostic target in preliminary radioimmunotherapeutic applications. Delta-like ligand 3 (DLL3), a molecule associated with PC switching toward neuroendocrine differentiation, was also successfully imaged via immunoPET with 89Zr-labeled antibodies. Other biomarkers, among whom vascular endothelial growth factor receptor 2 (VEGFR-2) and CD46, were also investigated through immunoPET in pre-clinical studies.
Conclusion
ImmunoPET pre-clinical studies have identified several biomarkers with potentially high impact on PC theranostics.

Similar content being viewed by others
Availability of data and material
Not applicable.
References
Evangelista L, Maurer T, van der Poel H et al (2022) [68Ga]Ga-PSMA versus [18F]PSMA positron emission tomography/computed tomography in the staging of primary and recurrent prostate cancer. a systematic review of the literature. Eur Urol Oncol 5:273–282. https://doi.org/10.1016/j.euo.2022.03.004
Mei R, Farolfi A, Morigi JJ, Fanti S (2022) The role of prostate-specific membrane antigen PET/computed tomography in the management of prostate cancer patients: could we ask for more? Curr Opin Urol 32:269–276. https://doi.org/10.1097/MOU.0000000000000982
Mokoala K, Lawal I, Lengana T et al (2021) PSMA theranostics: science and practice. Cancers 13:3904. https://doi.org/10.3390/cancers13153904
Filippi L, Chiaravalloti A, Schillaci O, Bagni O (2020) The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev Anticancer Ther 20:823–829. https://doi.org/10.1080/14737140.2020.1814151
Alberts I, Sachpekidis C, Fech V et al (2020) PSMA-negative prostate cancer and the continued value of choline-PET/CT. Nuklearmedizin 59:33–34. https://doi.org/10.1055/a-1044-1855
Cytawa W, Kircher S, Kübler H et al (2022) Diverse PSMA expression in primary prostate cancer: reason for negative [68Ga]Ga-PSMA PET/CT scans? Immunohistochemical validation in 40 surgical specimens. Eur J Nucl Med Mol Imagin. https://doi.org/10.1007/s00259-022-05831-8
Maraj B, Markham A (1999) Prostate-specific membrane antigen (FOLH1): recent advances in characterising this putative prostate cancer gene. Prostate Cancer Prostatic Dis 2:180–185. https://doi.org/10.1038/sj.pcan.4500325
Bakht MK, Derecichei I, Li Y et al (2019) Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr Relat Cancer 26:131–146. https://doi.org/10.1530/ERC-18-0226
Emmett L, Willowson K, Violet J et al (2017) Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci 64:52–60. https://doi.org/10.1002/jmrs.227
Sgouros G, Dewaraja YK, Escorcia F et al (2021) Tumor response to radiopharmaceutical therapies: the knowns and the unknowns. J Nucl Med 62:12S-22S. https://doi.org/10.2967/jnumed.121.262750
Gillessen S, Armstrong A, Attard G et al (2022) Management of patients with advanced prostate cancer: report from the advanced prostate cancer consensus conference 2021. Eur Urol 82:115–141. https://doi.org/10.1016/j.eururo.2022.04.002
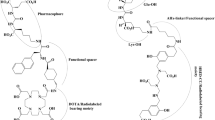
Wei W, Rosenkrans ZT, Liu J et al (2020) ImmunoPET: concept, design, and applications. Chem Rev 120:3787–3851. https://doi.org/10.1021/acs.chemrev.9b00738
Pool M, Kol A, de Jong S et al (2017) 89 Zr-mAb3481 PET for HER3 tumor status assessment during lapatinib treatment. mAbs 9:1370–1378. https://doi.org/10.1080/19420862.2017.1371382
Stoddart A (2016) Molecular imaging: seeing the target. Nat Rev Mater 1:16057. https://doi.org/10.1038/natrevmats.2016.57
Filippi L, Valentini FB, Gossetti B et al (2005) Intraoperative gamma probe detection of head and neck paragangliomas with 111 in-pentetreotide: a pilot study. Tumori 91:173–176. https://doi.org/10.1177/030089160509100213
Malmberg J, Tolmachev V, Orlova A (2011) Imaging agents for in vivo molecular profiling of disseminated prostate cancer: cellular processing of [111In]-labeled CHX-A″DTPA-trastuzumab and anti-HER2 ABY-025 Affibody in prostate cancer cell lines. Exp Ther Med 2:523–528. https://doi.org/10.3892/etm.2011.217
Mendoza N, Phillips GL, Silva J et al (2002) Inhibition of ligand-mediated HER2 activation in androgen-independent prostate cancer. Cancer Res 62:5485–5488
Potamianos S, Varvarigou AD, Archimandritis SC (2000) Radioimmunoscintigraphy and radioimmunotherapy in cancer: principles and application. Anticancer Res 20:925–948
Filippi L, Schillaci O (2006) SPECT/CT with a hybrid camera: a new imaging modality for the functional anatomical mapping of infections. Expert Rev Med Devices 3:699–703. https://doi.org/10.1586/17434440.3.6.699
Chacko A-M, Li C, Nayak M et al (2014) Development of 124 I Immuno-PET Targeting Tumor Vascular TEM1/Endosialin. J Nucl Med 55:500–507. https://doi.org/10.2967/jnumed.113.121905
van Dongen GAMS, Beaino W, Windhorst AD et al (2021) The role of 89 Zr-Immuno-PET in navigating and derisking the development of biopharmaceuticals. J Nucl Med 62:438–445. https://doi.org/10.2967/jnumed.119.239558
Yoon J-K, Park B-N, Ryu E-K et al (2020) Current perspectives on 89Zr-PET imaging. IJMS 21:4309. https://doi.org/10.3390/ijms21124309
Dijkers ECF, Kosterink JGW, Rademaker AP et al (2009) Development and characterization of clinical-grade 89 Zr-Trastuzumab for HER2/ neu ImmunoPET imaging. J Nucl Med 50:974–981. https://doi.org/10.2967/jnumed.108.060392
Oosting SF, van Asselt SJ, Brouwers AH et al (2016) 89 Zr-bevacizumab PET visualizes disease manifestations in patients with von hippel-lindau disease. J Nucl Med 57:1244–1250. https://doi.org/10.2967/jnumed.115.167643
Makris NE, Boellaard R, van Lingen A et al (2015) PET/CT-derived whole-body and bone marrow dosimetry of 89 Zr-Cetuximab. J Nucl Med 56:249–254. https://doi.org/10.2967/jnumed.114.147819
Pandit-Taskar N, O’Donoghue JA, Beylergil V et al (2014) 89Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imag 41:2093–2105. https://doi.org/10.1007/s00259-014-2830-7
Pandit-Taskar N, O’Donoghue JA, Ruan S et al (2016) First-in-human imaging with 89 Zr-Df-IAB2M anti-psma minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med 57:1858–1864. https://doi.org/10.2967/jnumed.116.176206
Joraku A, Hatano K, Kawai K et al (2019) Phase I/IIa PET imaging study with 89zirconium labeled anti-PSMA minibody for urological malignancies. Ann Nucl Med 33:119–127. https://doi.org/10.1007/s12149-018-1312-6
Frigerio B, Morlino S, Luison E et al (2019) Anti-PSMA 124I-scFvD2B as a new immuno-PET tool for prostate cancer: preclinical proof of principle. J Exp Clin Cancer Res 38:326. https://doi.org/10.1186/s13046-019-1325-6
Carmon KS, Azhdarinia A (2018) Application of immuno-PET in antibody-drug conjugate development. Mol Imag 17:153601211880122. https://doi.org/10.1177/1536012118801223
Verel I, Visser GWM, van Dongen GA (2005) The promise of immuno-PET in radioimmunotherapy. J Nucl Med 46(Suppl 1):164S-S171
Tateishi U, Daisaki H, Tsuchiya J et al (2021) Image quality and quantification accuracy dependence on patient body mass in 89Zr PET/CT imaging. EJNMMI Phys 8:72. https://doi.org/10.1186/s40658-021-00420-4
Saeki N, Gu J, Yoshida T, Wu X (2010) Prostate stem cell antigen: a jekyll and hyde molecule? Fig. 1. Clin Cancer Res 16:3533–3538. https://doi.org/10.1158/1078-0432.CCR-09-3169
Han K-R, Seligson DB, Liu X et al (2004) Prostate stem cell antigen expression is associated with gleason score, seminal vesicle invasion and capsular invasion in prostate cancer. J Urol 171:1117–1121. https://doi.org/10.1097/01.ju.0000109982.60619.93
Moore ML, Teitell MA, Kim Y et al (2008) Deletion of PSCA increases metastasis of TRAMP-Induced prostate tumors without altering primary tumor formation. Prostate 68:139–151. https://doi.org/10.1002/pros.20686
Gu Z, Thomas G, Yamashiro J et al (2000) Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 19:1288–1296. https://doi.org/10.1038/sj.onc.1203426
Knowles SM, Zettlitz KA, Tavaré R et al (2014) Quantitative ImmunoPET of prostate cancer xenografts with 89 Zr- and 124 I-Labeled Anti-PSCA A11 minibody. J Nucl Med 55:452–459. https://doi.org/10.2967/jnumed.113.120873
Knowles SM, Tavaré R, Zettlitz KA et al (2014) Applications of ImmunoPET: using 124 I-Anti-PSCA A11 minibody for imaging disease progression and response to therapy in mouse xenograft models of prostate cancer. Clin Cancer Res 20:6367–6378. https://doi.org/10.1158/1078-0432.CCR-14-1452
Tsai WK, Zettlitz KA, Tavaré R et al (2018) Dual-modality ImmunoPET/Fluorescence imaging of prostate cancer with an Anti-PSCA Cys-Minibody. Theranostics 8:5903–5914. https://doi.org/10.7150/thno.27679
Cacciamani GE, Shakir A, Tafuri A et al (2020) Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: a systematic review-based expert consensus. World J Urol 38:883–896. https://doi.org/10.1007/s00345-019-02870-z
Zettlitz KA, Tsai W-TK, Knowles SM et al (2018) Dual-modality Immuno-PET and near-infrared fluorescence imaging of pancreatic cancer using an anti-prostate stem cell antigen cys-diabody. J Nucl Med 59:1398–1405. https://doi.org/10.2967/jnumed.117.207332
Tsai W-TK, Zettlitz KA, Dahlbom M et al (2020) Evaluation of [131I]I- and [177Lu]Lu-DTPA-A11 minibody for radioimmunotherapy in a preclinical model of PSCA-expressing prostate cancer. Mol Imag Biol 22:1380–1391. https://doi.org/10.1007/s11307-020-01518-4
Kramer CS, Dimitrakopoulou-Strauss A (2022) Immuno-imaging (PET/SPECT)–quo vadis? Molecules 27:3354. https://doi.org/10.3390/molecules27103354
Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11:69. https://doi.org/10.1038/s41408-021-00459-7
Adkins RN, MSN, ANP-C S, (2019) CAR T-cell therapy: adverse events and management. JADPRO 10. https://doi.org/10.6004/jadpro.2019.10.4.11
Koristka S, Kegler A, Bergmann R et al (2018) Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J Autoimmun 90:116–131. https://doi.org/10.1016/j.jaut.2018.02.006
Arndt C, Bergmann R, Striese F et al (2022) Development and functional characterization of a versatile radio-/immunotheranostic tool for prostate cancer management. Cancers 14:1996. https://doi.org/10.3390/cancers14081996
Nijhout HF (2003) Development and evolution of adaptive polyphenisms. Evol Dev 5:9–18. https://doi.org/10.1046/j.1525-142X.2003.03003.x
Davies AH, Beltran H, Zoubeidi A (2018) Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol 15:271–286. https://doi.org/10.1038/nrurol.2018.22
Conteduca V, Oromendia C, Eng KW et al (2019) Clinical features of neuroendocrine prostate cancer. Eur J Cancer 121:7–18. https://doi.org/10.1016/j.ejca.2019.08.011
Sharma SK, Pourat J, Abdel-Atti D et al (2017) Noninvasive interrogation of DLL3 expression in metastatic small cell lung cancer. Cancer Res 77:3931–3941. https://doi.org/10.1158/0008-5472.CAN-17-0299
Korsen JA, Kalidindi TM, Khitrov S et al (2022) Molecular imaging of neuroendocrine prostate cancer by targeting delta-like ligand 3. J Nucl Med Jnumed 121:263221. https://doi.org/10.2967/jnumed.121.263221
Mojtahedi A, Thamake S, Tworowska I et al (2014) The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imag 4:426–434
Filippi L, Scopinaro F, Pelle G et al (2016) Molecular response assessed by 68Ga-DOTANOC and survival after 90Y microsphere therapy in patients with liver metastases from neuroendocrine tumours. Eur J Nucl Med Mol Imag 43:432–440. https://doi.org/10.1007/s00259-015-3178-3
Takahashi H, Shibuya M (2005) The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci 109:227–241. https://doi.org/10.1042/CS20040370
Roberts E, Cossigny DAF, Quan GMY (2013) The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer 2013:1–8. https://doi.org/10.1155/2013/418340
Li M, Jiang D, Barnhart TE et al (2019) Immuno-PET imaging of VEGFR-2 expression in prostate cancer with 89Zr-labeled ramucirumab. Am J Cancer Res 9:2037–2046
Persson BD, Schmitz NB, Santiago C et al (2010) Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog 6:e1001122. https://doi.org/10.1371/journal.ppat.1001122
Wang S, Li J, Hua J et al (2021) Molecular imaging of prostate cancer targeting CD46 using ImmunoPET. Clin Cancer Res 27:1305–1315. https://doi.org/10.1158/1078-0432.CCR-20-3310
Lenárt S, Lenárt P, Šmarda J et al (2020) Trop2: jack of all trades, master of none. Cancers 12:3328. https://doi.org/10.3390/cancers12113328
Hsu E-C, Rice MA, Bermudez A et al (2020) Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci USA 117:2032–2042. https://doi.org/10.1073/pnas.1905384117
van Rij CM, Frielink C, Goldenberg DM et al (2015) Pretargeted ImmunoPET of prostate cancer with an anti-TROP-2 x anti-hsg bispecific antibody in mice with PC3 xenografts. Mol Imaging Biol 17:94–101. https://doi.org/10.1007/s11307-014-0772-x
Altai M, Membreno R, Cook B et al (2017) Pretargeted imaging and therapy. J Nucl Med 58:1553–1559. https://doi.org/10.2967/jnumed.117.189944
Oliveira MC, Correia JDG (2022) Clinical application of radioiodinated antibodies: where are we? Clin Transl Imag 10:123–162. https://doi.org/10.1007/s40336-021-00477-2
Zhang Z, Li D, Yun H et al (2022) CAR-T Cells in the treatment of urologic neoplasms: present and future. Front Oncol 12:915171. https://doi.org/10.3389/fonc.2022.915171
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
All the authors equally contributed to conception and design of the article, or acquisition, analysis and interpretation of data; L.F. and L.E. wrote the initial draft of the manuscript; M.M.S. and O.S. critically revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Filippi, L., Evangelista, L., Sathekge, M.M. et al. ImmunoPET for prostate cancer in the PSMA era: do we need other targets?. Clin Transl Imaging 10, 587–596 (2022). https://doi.org/10.1007/s40336-022-00520-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-022-00520-w