Abstract
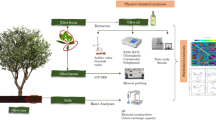
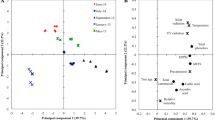
The olive species (Olea europaea L.) is an ancient traditional crop grown under rainfed conditions in the Mediterranean Basin. In response to the growing national and international demand for olive oil, the olive cultivars are introduced into highly arid new bioclimatic areas. Subsequently, the morpho-physiology and phytochemistry of olive trees are potentially changing among cultivar types and geographical conditions. In the present work, we have undertaken an assessment on the impacts of geographical location and cultivar types on the leaf morpho-physiology and phytochemistry of olive trees. Thus, leaves of the two most cultivated olive tree varieties, Chemlal and Sigoise, were collected from three geographical regions (Setif, Batna, and Eloued) with increasing aridity in Algeria. Leaf samples from the geographical regions were analyzed using the standard physiological experiment, colorimetric method, and a chromatography assay. Leaves of both cultivars exhibited a significant variance in terms of the leaf shape index but not for the leaf tissue density, specific leaf weight, and specific leaf area. Photosynthetic pigment contents were affected by both cultivar type and geographical location, with the lowest pigment content recorded in the Sigoise cultivar from the Setif region. Compared with the Setif and Batna regions, dried leaves of both cultivars from the Eloued region showed the higher levels of the total polyphenol, total flavonoid, and total tannin, as well as a better antioxidant capacity. Liquid chromatography-mass spectrometry analysis of all leaf extracts identified the following phenolic acids as major compounds: oleuropein, naringin, apigenin-7-O-glucoside, kaempferol, quercetin, quercitrin, luteolin-7-O-naringenin, and quinic acid. Lower contents were found for p-Coumaric acid, trans-Ferulic acid, hyperoside, rutin, apigenin, caffeic acid, protocatechuic acid, o-Coumaric acid, and gallic acid. Also, epicatechin and catechin+ were not found in the leaf extracts of the Sigoise cultivar. The leaf organic extracts in both cultivars displayed promising anti-cancer activity that was affected by geographical location and organic solvent polarity. Briefly, although increasing aridity and soil organic and mineral deficiency affected the leaf morpho-physiological parameters, both cultivars sustained a chemical richness, a good antioxidant, and an anti-tumoral capacity in leaves. Furthermore, the findings revealed that regardless the olive tree genotype, there was a significant impact of geographical location on the leaf morpho-physiology, bioactivity, and chemical composition, which may consequently modulate the growth and oil production of olive trees.
Similar content being viewed by others
References
Ababsa N, Kribaa M, Tamrabet L, et al. (2020). Long-term effects of wastewater reuse on hydro physicals characteristics of grassland grown soil in semi-arid Algeria. Journal of King Saud University-Science, 32(1): 1004–1013.
Abaza L, Talorete T P N, Yamada P, et al. (2007). Tunisian Gerboui olive leaf extract induces growth inhibition and differentiation of human leukemia HL-60 cells. Journal Bioscience Biotechnology and Biochemistry, 71(5): 1306–1312.
Abdessemed S, Abdessemed A, Boudchicha R H, et al. (2018). Characterization and identification of some olive ecotypes (Olea europaea L.) in Algeria. Agriculture Journal, 8(2): 26–43. (in French)
Acar-Tek N, Ağagündüz D. (2020). Olive leaf (Olea europaea L. folium): Potential effects on glycemia and lipidemia. Annals of Nutrition and Metabolism, 76(1): 10–15.
Ahmad-Qasem M H, Cánovas J, Barrajón-Catalán E, et al. (2013). Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innovative Food Science and Emerging Technologies, 17: 120–129.
Aïachi Mezghani M, Laaribi I, Zouari I, et al. 2021. Sustainability and plasticity of the olive tree cultivation in arid conditions. In: Khebour Allouche F, Abu-hashim M, Negm A M. Agriculture Productivity in Tunisia under Stressed Environment. Springer Water. Cham: Springer, 27–56.
Al Juhaimi F, Özcan M M, Uslu N, et al. (2018). The effects of conventional heating on phenolic compounds and antioxidant activities of olive leaves. Journal of Food Science and Technology, 55: 4204–4211.
Antoniou C, Hull J. (2021). The Anti-cancer effect of Olea europaea L. products: a review. Current Nutrition Reports, 10(1): 99–124.
Bacelar E A, Correia C M, Moutinho-Pereira J M, et al. (2004). Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiology, 24(2): 233–239.
Bacelar E A, Santos D L, Moutinho-Pereira J M, et al. (2006). Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: Changes on structure and chemical composition of foliage and oxidative damage. Plant Science, 170(3): 596–605.
Bahloul N, Kechaou N, Mihoubi N B. (2014). Comparative investigation of minerals, chlorophylls contents, fatty acid composition and thermal profiles of olive leaves (Olea europaea L.) as by-product. Grasas y Aceites, 65(3): e035, doi: https://doi.org/10.3989/gya.0102141.
Bekir J, Mars M, Souchard J P, et al. (2013). Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food and Chemical Toxicology, 55: 470–475.
Benhammou N, Bekkara F A, Panovska T K. (2009). Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. Comptes Rendus Chimie, 12(12): 1259–1266.
Bilgin M, Şahin S. (2013). Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. Journal of the Taiwan Institute of Chemical Engineers, 44(1): 8–12.
Bona E, Massa N, Toumatia O, et al. (2021). Climatic zone and soil properties determine the biodiversity of the soil bacterial communities associated to native plants from desert areas of North-Central Algeria. Microorganisms, 9(7): 1359, doi: https://doi.org/10.3390/microorganisms9071359.
Borjan D, Leitgeb M, Knez Ž, et al. (2020). Microbiological and antioxidant activity of phenolic compounds in olive leaf extract. Molecules, 25(24): 5946, doi: https://doi.org/10.3390/molecules25245946.
Brahim N, Karbout N, Dhaouadi L, et al. (2021). Global landscape of organic carbon and total nitrogen in the soils of oasis ecosystems in southern Tunisia. Agronomy, 11(10): 1903, doi: https://doi.org/10.3390/agronomy11101903.
Brahmi F, Mechri B, Dabbou S, et al. (2012). The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Industrial Crops and Products, 38: 146–152.
Condelli N, Caruso M C, Galgano F, et al. (2015). Prediction of the antioxidant activity of extra virgin olive oils produced in the Mediterranean area. Food Chemistry, 177: 233–239.
Connor D J. (2005). Adaptation of olive (Olea europaea L.) to water-limited environments. Australian Journal of Agricultural Research, 56(11): 1181–1189.
Connor D J, Fereres E. (2010). The physiology of adaptation and yield expression in olive. Horticultural Reviews, 31: 155–229.
Corona G, Deiana M, Incani A, et al. (2009). Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Molecular Nutrition and Food Research, 53(7): 897–903.
Datt B. (1999). Remote sensing of water content in Eucalyptus leaves. Australian Journal of Botany, 47(6): 909–923.
de Bock M, Thorstensen E B, Derraik J G, et al. (2013). Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Molecular Nutrition and Food Research, 57(11): 2079–2085.
de Marino S, Festa C, Zollo F, et al. (2014). Antioxidant activity and chemical components as potential anticancer agents in the olive leaf (Olea europaea L. cv Leccino.) decoction. Anti-Cancer Agents in Medicinal Chemistry, 14(10): 1376–1385.
Dhia G, Benchaben H, Abdelkrim H. (2018). Foliar behavior of olive trees (Olea europaea L.) grafted and cut under the effect of salt stress. Ukrainian Journal of Ecology, 8(1): 578–584.
di Donna L, Mazzotti F, Naccarato A, et al. (2010). Secondary metabolites of Olea europaea leaves as markers for the discrimination of cultivars and cultivation zones by multivariate analysis. Food Chemistry, 121(2): 492–496.
Diaz-Espejo A, Buckley T N, Sperry J S, et al. (2012). Steps toward an improvement in process-based models of water use by fruit trees: A case study in olive. Agricultural Water Management, 114: 37–49.
Douzane M, Daas M S, Meribai A, et al. (2021). Physico-chemical and sensory evaluation of virgin olive oils from several Algerian olive-growing regions. Oilseeds and Fats, Crops and Lipids, 28: 55, doi: https://doi.org/10.1051/ocl/2021044.
Ennajeh M, Vadel A M, Cochard H, et al. (2010). Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. Journal of Horticultural Science and Biotechnology, 85(4): 289–294.
Georgé S, Brat P, Alter P, et al. (2005). Rapid determination of polyphenols and vitamin C in plant-derived products. Journal of Agricultural and Food Chemistry, 53(5): 1370–1373.
Gharibi S, Tabatabaei B E S, Saeidi G, et al. (2019). The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry, 162: 90–98.
Gilani A H, Khan A U. 2010. Medicinal value of combination of cholinergic and calcium antagonist constituents in olives. In: Preedy V R, Watson R R. Olives and Olive Oil in Health and Disease Prevention. Amsterdam: Elsevier, 835–843.
Goldsmith C D, Vuong Q V, Sadeqzadeh E, et al. (2015). Phytochemical properties and anti-proliferative activity of Olea europaea L. leaf extracts against pancreatic cancer cells. Molecules, 20(7): 12992–13004.
Gomes S, Martins-Lopes P, Guedes-Pinto H. 2012. Olive tree genetic resources characterization through molecular markers. In: Caliskan M. Genetic Diversity in Plants. Croatia: InTech Publisher, 15–28.
Guerfel M, Baccouri O, Boujnah D, et al. (2009). Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Scientia Horticulturae, 119(3): 257–263.
Guinda Á, Castellano J M, Santos-Lozano J M, et al. (2015). Determination of major bioactive compounds from olive leaf. LWT — Food Science and Technology, 64(1): 431–438.
Hashim Y Z Y, Rowland I R, McGlynn H, et al. (2008). Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. International Journal of Cancer, 122(3): 495–500.
Hassen I, Casabianca H, Hosni K. (2015). Biological activities of the natural antioxidant oleuropein: Exceeding the expectation — A mini-review. Journal of Functional Foods, 18: 926–940.
Ilarioni L, Proietti P. (2014). Olive tree cultivars. In: Peri C. The Extra-Virgin Olive Oil Handbook. Oxford: Wiley, 5: 59–67.
IOC (International Olive Council). 1997. Methodology for the primary characterization of olive cultivars. In: RESGEN CT Project (67/97). European Union, IOC. Madrid, Spain.
Jones R J, Palmer B. (2000). In vitro digestion studies using 14C-labelled polyethylene glycol (PEG) 4000: comparison of six tanniniferous shrub legumes and the grass Panicum maximum. Animal Feed Science and Technology, 85(3–4): 215–221.
Karonen M, Oraviita M, Mueller-Harvey I, et al. (2015). Binding of an oligomeric ellagitannin series to bovine serum albumin (BSA): Analysis by isothermal titration calorimetry (ITC). Journal of Agricultural and Food Chemistry, 63(49): 10647–10654.
Kermanshah Z, Samadanifard H, Moghaddam O M, et al. (2020). Olive leaf and its various health-benefitting effects: A review study. Pakistan Journal of Medical and Health Sciences, 14(2): 1301–1312.
Lichtenthaler H K, Buschmann C. 2001. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. In: Wrolstad R E, Acree T E, Decker E A. Current Protocols in Food and Analytical Chemistry. New York: Wiley, F.4.2.1–F.4.2.6.
Lins P G, Pugine S M P, Scatolini A M, et al. (2018). In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon, 4(9): e00805, doi: https://doi.org/10.1016/j.heliyon.2018.e00805.
Maayan I, Shaya F, Ratner K, et al. (2008). Photosynthetic activity during olive (Olea europaea) leaf development correlates with plastid biogenesis and Rubisco levels. Physiologia Plantarum, 134(3): 547–558.
Malterre H. 1961. Rational nomenclature of the textures of soils and loose or calcareous rocks. French Association for Soil Studies newsletter. France: Special Issue,, 27–40 (in Frensh).
Markhali F S, Teixeira J A, Rocha C M. (2020). Olive tree leaves a source of valuable active compounds. Processes, 8(9): 1177, doi: https://doi.org/10.3390/pr8091177.
Marsh K J, Wallis I R Kulheim C, et al. (2020). New approaches to tannin analysis of leaves can be used to explain in vitro biological activities associated with herbivore defence. New Phytologist, 225(1): 488–498.
Martín-García B, Verardo V, León L, et al. (2019). GC-QTOF-MS as valuable tool to evaluate the influence of cultivar and sample time on olive leaves triterpenic components. Food Research International, 115: 219–226.
Martín-García B, de Montijo-Prieto S, Jiménez-Valera M, et al. (2022). Comparative extraction of phenolic compounds from olive leaves using a sonotrode and an ultrasonic bath and the evaluation of both antioxidant and antimicrobial activity. Antioxidants, 11(3): 558, doi: https://doi.org/10.3390/antiox11030558.
Medina E, Romero C, García P, et al. (2019). Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions. Food and Function, 10(8): 4716–4724.
Mehalaine S, Chenchouni H. (2020). Plants of the same place do not have the same metabolic pace: soil properties affect differently essential oil yields of plants growing wild in semiarid Mediterranean lands. Arabian Journal of Geosciences, 13: 1263, doi: https://doi.org/10.1007/s12517-020-06219-4.
Miliauskas G, Venskutonis P R, van Beek T A. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry, 85(2): 231–237.
Mohamed M B, Gusami F, Ali S B, et al. (2018). The LC-MS/MS characterization of phenolic compounds in leaves allows classifying olive cultivars grown in South Tunisia. Biochemical Systematics and Ecology, 78: 84–90.
Moilanen J, Karonen M, Tähtinen P, et al. (2016). Biological activity of ellagitannins: Effects as anti-oxidants, pro-oxidants and metal chelators. Phytochemistry, 125: 65–72.
Muzzalupo I, Vendramin G G, Chiappetta A. (2014). Genetic biodiversity of Italian olives (Olea europaea) germplasm analyzed by SSR markers. The Scientific World Journal, 2014: 296590, doi: https://doi.org/10.1155/2014/296590.
Nelson D W, Sommers L E. 1983. Total carbon, organic carbon, and organic matter. In: Page A L, Miller R H, Keeney D R. Methods of Soil Analysis Part 2, Chemical and Microbiological Properties. Agronomy Monograph No. 9. Madison: American Society of Agronomy, 539–579.
Nelson R E. 1983. Carbonate and gypsum. In: Page A L, Miller R H, Keeney D R. Methods of Soil Analysis Part 2, Chemical and Microbiological Properties. Agronomy Monograph No. 9. Madison: American Society of Agronomy, 181–197.
Niyogi K K, Björkman O, Grossman A R. (1997). The roles of specific xanthophylls in photoprotection. Proceedings of the National Academy of Sciences, 94(25): 14162–14167.
Omar Z, Bouajila A, Brahim N, et al. (2017). Soil property and soil organic carbon pools and stocks of soil under oases in arid regions of Tunisia. Environmental Earth Sciences, 76: 415, doi: https://doi.org/10.1007/s12665-017-6745-z.
Ozkaya M T, Cakir E, Gokbayrak Z, et al. (2006). Morphological and molecular characterization of Derik Halhali olive (Olea europaea L.) accessions grown in Derik-Mardin province of Turkey. Scientia Horticulturae, 108(2): 205–209.
Parida A K, Dagaonkar V S, Phalak M S, et al. (2007). Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnology Reports, 1: 37–48.
Pereira A P, Ferreira I C F R, Marcelino F, et al. (2007). Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules, 12(5): 1153–1162.
Petridis A, Therios I, Samouris G. (2012). Genotypic variation of total phenol and oleuropein concentration and antioxidant activity of 11 Greek olive cultivars (Olea europaea L.). HortScience, 47(3): 339–342.
Pita P, Pardos J A. (2001). Growth, leaf morphology, water use and tissue water relations of Eucalyptus globulus clones in response to water deficit. Tree Physiology, 21(9): 599–607.
Rahmani R, Beaufort S, Villarreal-Soto S A, et al. (2019). Kombucha fermentation of African mustard (Brassica tournefortii) leaves: chemical composition and bioactivity. Food Bioscience, 30: 100414, doi: https://doi.org/10.1016/j.fbio.2019.100414.
Robinson G W. (1922). A new method for the mechanical analysis of soils and other dispersions. The Journal of Agricultural Science, 12(3): 306–321.
Ruzzolini J, Peppicelli S, Andreucci E, et al. (2018). Oleuropein, the main polyphenol of Olea europaea leaf extract, has an anti-cancer effect on human BRAF melanoma cells and potentiates the cytotoxicity of current chemotherapies. Nutrients, 10(12): 1950, doi: https://doi.org/10.3390/nu10121950.
Salah M B, Abdelmelek H, Abderraba M. (2012). Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Medicinal Chemistry, 2(5): 107–111.
Sarker U, Oba S. (2018). Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biology, 18(1): 258, doi: https://doi.org/10.1186/s12870-018-1484-1.
Schofield P, Mbugua D M, Pell A N. (2001). Analysis of condensed tannins: A review. Animal Feed Science and Technology, 91(1–2): 21–40.
Shaheen M A, Hegazi A A, Hmmam I S A. (2011). Effect of water stress on vegetative characteristics and leaves chemical constituents of some transplants olive cultivars. American-Eurasian Journal of Agricultural and Environmental Sciences, 11(5): 663–670.
Singleton V L, Rossi J A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16: 144–158.
Talhaoui N, Taamalli A, Gomez-Caravaca A M, et al. (2015). Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Research International, 77: 92–108.
Tekaya M, El-Gharbi S, Mechri B, et al. (2016). Improving performance of olive trees by the enhancement of key physiological parameters of olive leaves in response to foliar fertilization. Acta Physiologiae Plantarum, 38: 101, doi: https://doi.org/10.1007/s11738-016-2122-x.
Terzuoli E, Giachetti A, Ziche M, et al. (2016). Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Molecular Nutrition and Food Research, 60(3): 519–529.
Tognetti R, Giovannelli A, Lavini A, et al. (2009). Assessing environmental controls over conductances through the soil-plant-atmosphere continuum in an experimental olive tree plantation of southern Italy. Agricultural and Forest Meteorology, 149(8): 1229–1243.
Vinha A F, Ferreres F, Silva B M, et al. (2005). Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chemistry, 89(4): 561–568.
Walkley A, Black I A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1): 29–38.
Wang B, Qu J, Feng S, et al. (2019). Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods, 8(12): 657, doi: https://doi.org/10.3390/foods8120657.
Zaanouni N, Gharssallaoui M, Eloussaief M, et al. (2018). Heavy metals transfer in the olive tree and assessment of food contamination risk. Environmental Science and Pollution Research, 25: 18320–18331.
Zeitoun M A M, Mansour M M, Ezzat S, et al. (2017). Effect of pretreatment of olive leaves on phenolic content and antioxidant activity. American Journal of Food Technology, 12(2): 132–139.
Zhishen J, Mengcheng T, Jianming W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64(4): 555–559.
Acknowledgements
We are grateful to Dr. Khaled JEBAHI from the English Language Institute, KING ABDULAZIZ University, Saudi Arabia for his effort on English editing of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Touati, S., Ayadi, J., Bouajila, A. et al. Leaf morpho-physiology and phytochemistry of olive trees as affected by cultivar type and increasing aridity. J. Arid Land 14, 1159–1179 (2022). https://doi.org/10.1007/s40333-022-0078-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-022-0078-9