Abstract
Background and Objective
Sodium valproate is a widely prescribed broad-spectrum antiepileptic drug. It shows high inter-individual variability in pharmacokinetics and pharmacodynamics and has a narrow therapeutic range. We evaluated the effects of polymorphic uridine diphosphate glucuronosyltransferase (UGT)1A6 (541A>G, 552A>C) metabolizing enzyme on the pharmacokinetics of sodium valproate in the patients with epilepsy who showed toxicity to therapy.
Methods
Genotype analysis of the patients was made with polymerase chain–restriction fragment length polymorphism (RFLP) with sequencing. Plasma drug concentrations were measured with reversed phase high-performance liquid chromatography (HPLC) and concentration–time data were analyzed by using a non-compartmental approach.
Results
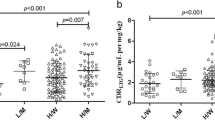
The results of this study suggested a significant genotypic as well as allelic association with valproic acid toxicity for UGT1A6 (541A>G) or UGT1A6 (552A>C) polymorphic enzymes. The elimination half-life (t ½ = 40.2 h) of valproic acid was longer and the clearance rate (CL = 917 ml/h) was lower in the poor metabolizers group of UGT1A6 (552A>C) polymorphism who showed toxicity than in the intermediate metabolizers group (t ½ = 35.5 h, CL = 1,022 ml/h) or the extensive metabolizers group (t ½ = 25.4 h, CL = 1,404 ml/h).
Conclusion
Our findings suggest that the UGT1A6 (552A>C) genetic polymorphism plays a significant role in the steady state concentration of valproic acid, and it thereby has an impact on the toxicity of the valproic acid used in the patients with epilepsy.


Similar content being viewed by others
References
Kotsopoulos IA, Van Merode T, Kessels FG, Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43:1402–9.
Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry. 1996;61:433–43.
St Louis EK. Minimizing AED adverse effects: improving quality of life in the interictal state in epilepsy care. Curr Neuropharmacol. 2009;7:106–14.
Evans WE, McLeod HL. Pharmacogenomics, drug disposition, drug targets, and side effects. N Engl J Med. 2003;384:538.
Chadwick DW. Concentration-effect relationships of sodium valproate. Clin. Pharmacokinet. 1985;10:155–63.
Baillie TL, Sheffels PR. Valproate: chemistry and biotransformation. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs. 4th ed. New York: Raven Press; 1995. p. 589–604.
Burchell B, Brierley CH, Rance D. Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation. Life Sci. 1995;57:1819–31.
King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1:143–61.
Burchell B, Brierley CH, Monaghan G. The structure and function of the UDP-glucuronosyltransferase gene family. Adv Pharmacol. 1998;42:335–8.
Mackenzie PI, Owens IS, Burchell B. The UDP-glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics. 1997;7:255–69.
Orzechowski A, Schrenk D, Bock-Hennig BS, Bock KW. Glucuronidation of carcinogenic arylamines and their N-hydroxy derivatives by rat and human phenol UDP-glucuronosyltransferase of the UGT1 gene complex. Carcinogenesis. 1994;15:1549–53.
Woosterk R, Sutherland L, Ebner T, Clarke D, Da Cruz e Silva O, Burchell B. Cloning and stable expression of a new member of the human liver phenol/ bilirubin: UDP-glucuronosyltransferase cDNA family. Biochem J. 1991;278:465–9.
Ciotti M, Marrone A, Potter C, Owens IS. Genetic polymorphism in the human UGT1A6 UDP-glucuronosyltransferase: pharmacological implications. Pharmacogenetics. 1997;7:485–95.
Krishnaswamy S, Hao Q, Al-Rohaimi A. UDP glucuronosyltransferase (UGT)1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S). J Pharmacol Exp Ther. 2005;313:1340–6.
Comey CT, Koons BW, Presley KW, Smerick JB, Sobieralski CA, Stanley DM. DNA extraction strategies for amplified fragment length polymorphism analysis. J Forensic Sci. 1994;39:1254–69.
Verlaan M, Morsche RH, Pap A, et al. Functional polymorphisms of UDP-glucuronosyltransferases 1A1, 1A6 and 1A8 are not involved in chronic pancreatitis. Pharmacogenetics. 2004;14:351–7.
Amini H, Javan M, Ahmadiani A. Development and validation of a sensitive assay of valproic acid in human plasma by high-performance liquid chromatography without prior derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:368–71.
Kishore P, Rajani Kumar V, Satyanarayana V, Krishna DR. HPLC determination of valproic acid in human serum. Pharmazie. 2003;58:378–80.
Ketter TA, Frye MA, Cora-Locatelli G, Kimbrell TA. Metabolism and excretion of mood stabilizers and new anticonvulsants. Cell Mol Neurobiol. 1999;19:511–32.
Nagar S, Zalatoris JJ, Blanchard RL. Human UGT 1A6 pharmacogenetics: identification of a novel SNP, characterization of allele frequencies and functional analysis of recombinant allozymes in human liver tissue and in cultured cells. Pharmacogenetics. 2004;14:487–99.
Lampe JW, Bigler J, Horner NK, Potter JD. UDP-glucuronosyltransferase (UGT1A1*28 and UGT1A6*2) polymorphisms in Caucasians and Asians: relationships to serum Bilirubin concentrations. Pharmacogenetics. 1999;9:341–9.
Zaccara G, Messori A, Moroni F. Clinical pharmacokinetics of valproic acid. Clin Pharmacokinet. 1998;15:367–89.
Park HM, Kang SS, Lee YB, Shin DJ, Kim ON, Lee SB, et al. Population pharmacokinetics of intravenous valproic acid in Korean patients. J Clin Pharm Ther. 2002;27:419–25.
Levy RH, Mattson RH, Meldrum BS, Perucca E. Antiepileptic drugs. 5th ed. New York: Raven Press; 2002.
Schobben F, van Der Kleijn E, Gabreels JM. Pharmacokinetics of di-n-propylacetate in epileptic patients. Eur J Clin Pharmacol. 1975;8:97–105.
Chu XM, Zhang LF, Wang GJ, Zhang SN, Zhou JH, Hao HP. Influence of UDP-glucuronosyltransferase polymorphisms on valproic acid pharmacokinetics in Chinese epilepsy patients. Eur J Clin Pharmacol. 2012;68:1395–401.
Wang Y, Gao L, Liu YP, Huang NN, Xu SJ, Ma DJ. Effect of UGTIA6 A541G genetic polymorphism on the metabolism of valproic acid in Han epileptic children from Henan. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:429–32.
Acknowledgments
The work was supported by the Department of Biotechnology (DBT), Ministry of Science and Technology, New Delhi, and we thank Issam Hamadeh, Pharm D, University of Florida, Gainesville for his valuable suggestions on the manuscript.
Conflicts of Interest
The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munisamy, M., Tripathi, M., Behari, M. et al. The Effect of Uridine Diphosphate Glucuronosyltransferase (UGT)1A6 Genetic Polymorphism on Valproic Acid Pharmacokinetics in Indian Patients with Epilepsy: A Pharmacogenetic Approach. Mol Diagn Ther 17, 319–326 (2013). https://doi.org/10.1007/s40291-013-0041-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-013-0041-8