Abstract
Objective
The objective of this observational study was to evaluate the safety and effectiveness of discharging stabilized neonates to complete their oral morphine weaning at home.
Study Design
This retrospective cohort study evaluated neonates treated with oral morphine at two hospitals in London, Ontario, Canada. Neonates who completed their morphine wean in hospital were compared with neonates who completed their morphine wean following discharge from hospital (at home).
Results
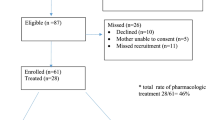
There were 80 neonates treated with oral morphine at two hospitals from 2006 to 2010. The majority (65 %, 52/80) of neonates completed their morphine weaning after hospital discharge and were significantly less likely to return to hospital for further withdrawal treatment (1/52 vs 4/28, p < 0.05). Neonates who were treated at home remained on morphine for more days (32 vs 19 days, p < 0.01).
Conclusions
We present the first North American cohort of neonates weaned with morphine at home for neonatal abstinence syndrome (NAS). We found that more days on oral morphine resulted in fewer returns to hospital for continued withdrawal management. There was no evidence of increased effectiveness, measured by the number of returns to hospital for further NAS management with in-hospital weaning. The estimated cost savings of continued weaning upon discharge was approximately $11,000 per patient (Canadian dollars). While further prospective research is necessary, in some cases morphine weaning at home may present a safe and cost-effective strategy for NAS management.

Similar content being viewed by others
References
Women ACoHCfU, American Society of Addiction M. ACOG Committee Opinion No. 524: opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–6.
Services USDoHaH. National Survey on Drug Use and Health: Summary of National Findings. 2010; Available from http://oas.samhsa.gov/NSDUH/2k10NSDUH/2k10Results.htm.
Pinto SM, Dodd S, Walkinshaw SA, Siney C, Kakkar P, Mousa HA. Substance abuse during pregnancy: effect on pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):137–41.
Seligman NS, Salva N, Hayes EJ, Dysart KC, Pequignot EC, Baxter JK. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol. 2008;199(4):396 e1–7.
Bateman BT, Hernandez-Diaz S, Rathmell JP, Seeger JD, Doherty M, Fischer MA, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014;120(5):1216–24.
de Castro A, Jones HE, Johnson RE, Gray TR, Shakleya DM, Huestis MA. Maternal methadone dose, placental methadone concentrations, and neonatal outcomes. Clin Chem. 2011;57(3):449–58.
Little BB, VanBeveren TT. Placental transfer of selected substances of abuse. Semin Perinatol. 1996;20(2):147–53.
Jansson LM, Di Pietro JA, Elko A, Williams EL, Milio L, Velez M. Pregnancies exposed to methadone, methadone and other illicit substances, and poly-drugs without methadone: a comparison of fetal neurobehaviors and infant outcomes. Drug Alcohol Depend. 2012;122(3):213–9.
O’Grady MJ, Hopewell J, White MJ. Management of neonatal abstinence syndrome: a national survey and review of practice. Arch Dis Child Fetal Neonatal Edition. 2009;94(4):F249–52.
Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol Off J Calif Perinat Assoc. 2006;26(1):15–7.
Agthe AG, Kim GR, Mathias KB, Hendrix CW, Chavez-Valdez R, Jansson L, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5):e849–56.
Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1–2):141–58.
Kelly JJ, Davis PG, Henschke PN. The drug epidemic: effects on newborn infants and health resource consumption at a tertiary perinatal centre. J Paediatr Child Health. 2000;36(3):262–4.
Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United states, 2000–2009. JAMA. 2012;307(18):1934–40.
Backes CH, Backes CR, Gardner D, Nankervis CA, Giannone PJ, Cordero L. Neonatal abstinence syndrome: transitioning methadone-treated infants from an inpatient to an outpatient setting. J Perinatol Off J Calif Perinatal Assoc. 2012;32(6):425–30.
Liu A, Bjorkman T, Stewart C, Nanan R. Pharmacological treatment of neonatal opiate withdrawal: between the devil and the deep blue sea. Int J Pediatr. 2011;2011:935631.
Kelly LE, Rieder MJ, Bridgman-Acker K, Lauwers A, Madadi P, Koren G. Are infants exposed to methadone in utero at an increased risk for mortality? J Popul Therap Clin Pharmacol. 2012;19(2):e160–5.
Fulroth R, Phillips B, Durand DJ. Perinatal outcome of infants exposed to cocaine and/or heroin in utero. Am J Dis Child. 1989;143(8):905–10.
Pritham UA. Breastfeeding promotion for management of neonatal abstinence syndrome. JOGNN/NAACOG. 2013;42(5):517–26.
Financial Disclosure
The authors of this article declare that they have no financial relationships to disclose pertinent to this article.
Conflict of interest
LE Kelly, D Knoppert, H Roukema, M Rieder and G Koren have no relevant conflicts of interest to declare.
Contributor’s Statement
Lauren Kelly is the first author who designed the study, collected and analyzed the data. David Knoppert was the pharmacy team lead and was responsible for overseeing the morphine-weaning calendars. Dr. Henry Roukema is a neonatologist directly involved in patient care at both centers. Dr. Michael Rieder and Dr. Gideon Koren were responsible for the study design, manuscript revisions and study oversight. All authors critically reviewed the manuscript and approved the final draft for submission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kelly, L.E., Knoppert, D., Roukema, H. et al. Oral Morphine Weaning for Neonatal Abstinence Syndrome at Home Compared with In-Hospital: An Observational Cohort Study. Pediatr Drugs 17, 151–157 (2015). https://doi.org/10.1007/s40272-014-0096-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-014-0096-y