Abstract
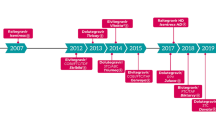
Integrase strand transfer inhibitors (INSTIs) bictegravir, dolutegravir, cabotegravir, elvitegravir and raltegravir, mostly available as fixed dose combinations are recommended treatments in both newly diagnosed and treatment-experienced patients with HIV. Their efficacy, good tolerability and simplified dosing regimens facilitate treatment adherence and a recently approved cabotegravir 4-weekly injection is a major advance.
Similar content being viewed by others
References
Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults. JAMA. 2020;324(16):1651–69.
Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2021. http://aidsinfo.nih.gov/. Accessed 27 May 2021.
Scarsi KK, Havens JP, Podany AT, et al. HIV-1 integrase inhibitors: a comparative review of efficacy and safety. Drugs. 2020;80(16):1649–76.
Viiv Healthcare. CABENUVA (cabotegravir and rilpivirine injectable suspension): US prescribing information. 2021. https://gskpro.com/. Accessed 27 May 2021.
ViiV Healthcare. Tivicay® (dolutegravir tablets or suspension): US prescribing information. 2021. https://gskpro.com/. Accessed 27 May 2021.
Gilead Sciences. Stribild® (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate tablets): US prescribing information. 2020. https://www.gilead.com/. Accessed 27 May 2021.
Gilead Sciences. Biktarvy® (bictegravir, emtricitabine, and tenofovir alafenamide tablets): US prescribing information. 2021. https://www.gilead.com/. Accessed 27 May 2021.
Orkin C, Arasteh K, Gorgolas Hernandez-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–35.
Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382(12):1112–23.
Viiv Healthcare. VOCABRIA (cabotegravir tablets): US prescribing information. 2021. https://gskpro.com/. Accessed 27 May 2021.
Viiv Healthcare. Dovato® (dolutegravir and lamivudine tablets): US prescribing information. 2021. https://gskpro.com/. Accessed 27 May 2021.
Viiv Healthcare. Juluca® (dolutegravir and rilpivirine tablets): US prescribing information. 2021. https://gskpro.com/. Accessed 27 May 2021.
Viiv Healthcare. Triumeq® (abacavir, dolutegravir, and lamivudine tablets): US prescribing information. 2020. https://gskpro.com/. Accessed 27 May 2021.
World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. 2019. https://www.who.int/. Accessed 27 May 2021.
Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–55.
Gilead Sciences. Genvoya® (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide tablets): US prescribing information. 2021. https://www.gilead.com/. Accessed 27 May 2021.
Merck & Co. Isentress® (raltegravir tablets or suspension): US prescribing information. 2021. https://www.merck.com/. Accessed 27 May 2021.
Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2019;71(6):1379–89.
Kolakowska A, Maresca AF, Collins IJ, et al. Update on adverse effects of HIV integrase inhibitors. Curr Treat Options Infect Dis. 2019;11(4):372–87.
Lepik KJ, Yip B, Ulloa AC, et al. Adverse drug reactions to integrase strand transfer inhibitors. AIDS. 2018;32:903–12.
Greenberg L, Ryom L, Wandeler G, et al. Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. JAIDS J Acquir Immune Defic Syndr. 2020;83(3):240–50.
Cuzin L, Pugliese P, Katlama C, et al. Integrase strand transfer inhibitors and neuropsychiatric adverse events in a large prospective cohort. J Antimicrob Chemother. 2019;74:754–60.
Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–72.
Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355–63.
Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–82.
Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364–72.
Molina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357–65.
Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347–56.
Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2020;74(12):3555–64.
Landovitz RJ, Li S, Grinsztejn B, Dawood H, Liu AY, Magnus M, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15(11):e1002690.
Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–40.
Overton ET, Richmond GJ, Rizzardini G, Jaeger H, Orrell C, Nagimova F et al. Cabotegravir + rilpivirine every 2 months is noninferior to monthly: ATLAS-2 M study [abstract no. 34]. In: Conference on Retroviruses and Opportunistic Infections (CROI). 2020.
Walmsley S, Baumgarten A, Berenguer J, et al. Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr. 2015;70(5):515–9.
Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–18.
Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–49.
Cahn P, Madero JS, Arribas JR, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;83(3):310–8.
Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–35.
Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–43.
Clotet B, Feinberg J, Van LJ, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–31.
Molina JM, Clotet B, Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127–36.
Sax PE, DeJesus E, Crofoot G, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–60.
Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381(9):827–40.
Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–8.
Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–62.
Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–48.
Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65(3):e118–20.
Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65(3):e121–4.
DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–38.
Rockstroh JK, DeJesus E, Henry K, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62(5):483–6.
Arribas JR, Thompson M, Sax PE, et al. Brief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment: week 144 results. J Acquir Immune Defic Syndr. 2017;75(2):211–8.
Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15.
Wohl D, Oka S, Clumeck N, et al. A randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. J Acquir Immune Defic Syndr. 2016;72(1):58–64.
Pozniak A, Flamm J, Antinori A, et al. Switching to the single-tablet regimen of elvitegravir, cobicistat, emtricitabine, and tenofovir DF from non-nucleoside reverse transcriptase inhibitor plus coformulated emtricitabine and tenofovir DF regimens: week 96 results of STRATEGY-NNRTI. HIV Clin Trials. 2017;18(4):141–8.
Pozniak A, Markowitz M, Mills A, et al. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis. 2014;14(7):590–9.
Arribas JR, DeJesus E, Lunzen J, et al. Simplification to single-tablet regimen of elvitegravir, cobicistat, emtricitabine, tenofovir DF from multi-tablet ritonavir-boosted protease inhibitor plus coformulated emtricitabine and tenofovir DF regimens: week 96 results of STRATEGY-PI. HIV Clin Trials. 2017;18(3):118–25.
Arribas JR, Pialoux G, Gathe J, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14(7):581–9.
Mills A, Crofoot G, Ortiz R, et al. Switching from twice-daily raltegravir plus tenofovir disoproxil fumarate/emtricitabine to once-daily elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate in virologically suppressed, HIV-1-infected subjects: 48 weeks data. HIV Clin Trials. 2014;15(2):51–6.
DeJesus E, Haas B, Segal-Maurer S, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res Hum Retroviruses. 2018;34(4):337–42.
Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52.
Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806.
Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77–85.
Eron JJ, Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11(12):907–15.
Cahn P, Kaplan R, Sax PE, et al. Raltegravir 1200 mg once daily versus raltegravir 400 mg twice daily, with tenofovir disoproxil fumarate and emtricitabine, for previously untreated HIV-1 infection: a randomised, double-blind, parallel-group, phase 3, non-inferiority trial. Lancet HIV. 2017;4(11):e486–94.
Cahn P, Sax PE, Squires K, et al. Raltegravir 1200 mg once daily vs 400 mg twice daily, with emtricitabine and tenofovir disoproxil fumarate, for previously untreated HIV-1 infection: week 96 results from ONCEMRK, a randomized, double-blind, noninferiority trial. J Acquir Immune Defic Syndr. 2018;78(5):589–98.
Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet. 2010;375(9712):396–407.
Eron JJ, Cooper DA, Steigbigel RT, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13(7):587–96.
Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–54.
Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatmentexperienced patients with drugresistant hiv infection: week 96 results of the benchmrk 1 and 2 phase III trials. Clin Infect Dis. 2010;50(4):605–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
C. Fenton, a contracted employee of Adis International Ltd/Springer Nature, and A. Lee, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. Z.T. Al-Salama is a salaried employee of Adis International/ Springer Nature, is an editor of Drugs & Therapy Perspectives, was not involved in any publishing decision for the manuscript, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Fenton, C., Lee, A. & Al-Salama, Z.T. Integrase strand transfer inhibitors can simplify HIV treatment. Drugs Ther Perspect 37, 300–312 (2021). https://doi.org/10.1007/s40267-021-00847-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00847-w