Abstract
Background
Despite the high risk of life-threatening complications due to drug-induced prolongation of the corrected QT interval (QTcP) in patients with end-stage renal disease (ESRD), the safety of QTcP-inducing drugs has rarely been investigated in this patient population.
Objectives
This study aimed to assess the appropriateness of prescriptions for QTcP-inducing drugs and evaluate potential drug–drug interactions (DDIs) in patients with ESRD in Jordan.
Methods
This study was a retrospective observational study conducted from October 2019 to March 2020 in the outpatient clinics of 36 healthcare facilities across Jordan. A standardized data collection form was developed and used by pharmacy staff to collect data from the electronic databases of these facilities. The targeted population was patients with ESRD, specifically their prescriptions. Micromedex Drug Reax® software was used to check potential DDIs. Data were analysed using SPSS v26 software.
Results
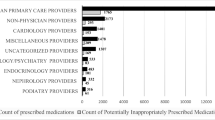
The study included 407 patients with ESRD, who were prescribed 954 drugs with a risk of inducing QTcP. Of these, 17.6% were considered inappropriate application, 12.9% inappropriate choice, and 26.4% inappropriate decision. Roughly two-thirds of the dispensed drugs (64.8%) were associated with a DDI, 10.4% of which were major, 29.3% moderate, and 60.3% minor. Predictors for major DDIs were major polypharmacy, type of clinic and geographic location. Predictors for inappropriate prescribing were type of clinic and geographic location.
Conclusion
High rates of DDIs and inappropriate prescribing of QTcP-inducing drugs were reported among patients with ESRD in outpatient clinics in Jordan.
Similar content being viewed by others
References
Liu P, Wang L, Han D, et al. Acquired long QT syndrome in chronic kidney disease patients. Ren Fail. 2020;42(1):54–65.
Patanè S, Marte F, Di Bella G, et al. QT interval prolongation, torsade de pointes and renal disease. Int J Cardiol. 2008;130(2):e71–3.
Alramly M, Darawad MW, Khalil AA. Slowing the progression of chronic kidney disease: comparison between predialysis and dialysis in Jordanian patients. Ren Fail. 2013;35(10):1348–52.
Sherif KA, Abo-Salem E, Panikkath R, et al. Cardiac repolarization abnormalities among patients with various stages of chronic kidney disease. Clin Cardiol. 2014;37(7):417–21.
Abdel-Qader DH, Al Meslamani AZ, Lewis PJ, et al. Incidence, nature, severity, and causes of dispensing errors in community pharmacies in Jordan. Int J Clin Pharm. 2020. https://doi.org/10.1007/s11096-020-01126-w.
Ibrahim OM, Ibrahim RM, Al Meslamani AZ, et al. Role of telepharmacy in pharmacist counselling to coronavirus disease 2019 patients and medication dispensing errors. J Telemed Telecare. 2020. https://doi.org/10.1177/1357633X20964347.
Abdel-Qader DH, Albassam A, Ismael NS, et al. Herbal medicine use in the Jordanian population: a nationally representative cross-sectional survey study design. J Pharm Pharmacogn Res. 2020;8(6):525–36.
Mohamed Ibrahim O, Ibrahim RM, Al Meslamani AZ, et al. Dispensing errors in community pharmacies in the United Arab Emirates: investigating incidence, types, severity, and causes. Pharm Pract (Granada). 2020;18(4):1–8.
Abdel-Qader DH, Al Meslamani AZ, El-Shara’ AA, et al. Investigating prescribing errors in the emergency department of a large governmental hospital in Jordan. J Pharm Heal Serv Res. 2020;11(4):375–82.
Mohamed Ibrahim O, Ibrahim RM, Abdel-Qader DH, et al. Evaluation of telepharmacy services in light of COVID-19. Telemed J E Health. 2020. https://doi.org/10.1089/tmj.2020.0283.
Al-Azzam SI, Abu-Dahoud EY, El-Khatib HA, et al. Etiologies of chronic renal failure in Jordanian population. J Nephrol. 2007;20(3):336–9.
Al-Niemat SI, Aljbouri TM. Antibiotic prescribing patterns in outpatient emergency clinics at Queen Rania Al Abdullah II Children’s Hospital, Jordan, 2013. Oman Med J. 2014;29(4):250–4.
Zalloum N, Farha R, Awwad O. Inappropriate prescribing of proton pump inhibitors among patients in two Jordanian tertiary health facilities. Trop J Pharm Res. 2016;15:1319–26.
Yasein N, Barghouti F, Irshaid Y. Elderly patients in family practice: polypharmacy and inappropriate prescribing: Jordan. Int Med J. 2012;19:115–26.
Al-Azayzih A, Gharaibeh S, Jarab AS, et al. Prevalence of Torsades de Pointes inducing drugs usage among elderly outpatients in North Jordan Hospitals. Saudi Pharm J. 2018;26(8):1146–54.
Hayes BD, Klein-Schwartz W, Barruetio F Jr. Polypharmacy and the geriatric patient. Clin Geriatr Med. 2007;23(2):371–90.
Patel VK, Acharya LD, Rajakannan T. Potential drug interactions in patients admitted to cardiology wards of a south Indian teaching hospital. Australas Med J. 2011;4(1):9–14.
Teixeira JJ, Crozatti MT, dos Santos CA, et al. Potential drug-drug interactions in prescriptions to patients over 45 years of age in primary care, southern Brazil. PLoS One. 2012;7(10):e47062.
Assefa S, Mekonen Z. Potential drug–drug interactions among adult patients admitted to medical wards at a tertiary teaching hospital in Ethiopia. J Drug Deliv Ther. 2018;8:348–54.
Wilcock A, Charlesworth S, Twycross R, et al. Prescribing non-opioid drugs in end-stage kidney disease. J Pain Symptom Manag. 2017;54(5):776–87.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82.
Allen LaPointe NM, Curtis LH, Chan KA, et al. Frequency of high-risk use of QT-prolonging medications. Pharmacoepidemiol Drug Saf. 2006;15(6):361–8.
Moreno-Gutiérrez PA, Gaviria-Mendoza A, Cañón MM, et al. High prevalence of risk factors in elderly patients using drugs associated with acquired torsades de pointes chronically in Colombia. Br J Clin Pharmacol. 2016;82(2):504–11.
Sarganas G, Garbe E, Klimpel A, et al. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16(1):101–8.
Das B, Rawat VS, Ramasubbu SK, et al. Frequency, characteristics and nature of risk factors associated with use of QT interval prolonging medications and related drug-drug interactions in a cohort of psychiatry patients. Therapie. 2019;74(6):599–609.
Danielsson B, Collin J, Jonasdottir Bergman G, et al. Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults: a Swedish nationwide study. Br J Clin Pharmacol. 2016;81(4):773–83.
AlRuthia Y, Alkofide H, Alosaimi FD, et al. Drug-drug interactions and pharmacists’ interventions among psychiatric patients in outpatient clinics of a teaching hospital in Saudi Arabia. Saudi Pharm J. 2019;27(6):798–802.
Ansari J. Drug interaction and pharmacist. J Young Pharm. 2010;2(3):326–31.
Alefan Q, Karasneh A, El-Dahiyat F, et al. Translation and validation of the Arabic version of generic medicines scale. Res Soc Adm Pharm. 2017;13(3):553–63.
Abdel-Qader DH, Albassam A, Ismael NS, et al. Community pharmacists’ knowledge of and attitudes toward antibiotic use, resistance, and self-medication in Jordan. Drugs Ther Perspect. 2020. https://doi.org/10.1007/s40267-020-00797-9.
Sommer J, Seeling A, Rupprecht H. Adverse drug events in patients with chronic kidney disease associated with multiple drug interactions and polypharmacy. Drugs Aging. 2020;37(5):359–72.
Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med. 2004;164(15):1621–5.
Acknowledgements
The authors thank Al-Balqà Applied University for facilitating this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to assist with the preparation of this manuscript
Conflict of interests
Ahmad Z. Al Meslamani, Dania Abu-Naser, Derar H. Abdel-Qader, Mohammed S. Aljamal, Mohammed A Alsharif, Mohamed Ahmed Mohammed Alshrahili, Nadia Al Mazrouei, and Osama Mohamed Ibrahim have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
Data are available upon reasonable request from the corresponding author.
Ethics Approval
The study was approved by the Ethics committee at Al Balqa University and the Ministry of Health.
Consent to participate
All participants completed a consent form indicating their willingness to participate.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conceptualization and design. AZM developed the study tool, concepts, data analysis, drafting the manuscript, and polishing the final version. DA and DAQ collected the data and participated in drafting and reviewing the manuscript. NA, OMA, MSJ, MAA and MAS wrote the first draft of the manuscript and participated in data analysis, and all authors commented on previous versions of it. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Al Meslamani, A.Z., Abu-Naser, D., Abdel-Qader, D.H. et al. Assessment of inappropriate prescribing of QT interval-prolonging drugs in end-stage renal disease patients in Jordan. Drugs Ther Perspect 37, 87–93 (2021). https://doi.org/10.1007/s40267-020-00806-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-020-00806-x