Abstract
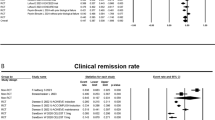
Oral tofacitinib (Xeljanz®) is a first-in-class, small molecule janus kinase (JAK) inhibitor that is approved for the treatment of moderate to severe ulcerative colitis in adults who have inadequately responded to, or are intolerant of, conventional therapy or a biological agent in the EU and in adults who have inadequately responded to, or are intolerant of, a tumour necrosis factor antagonist in the USA. In the pivotal phase 3 trials, tofacitinib induction (10 mg twice daily) and maintenance (5 or 10 mg twice daily) treatment significantly improved clinical and endoscopic findings of ulcerative colitis and health-related quality of life relative to placebo, with clinical benefits maintained longer term with flexible dosing strategy. Tofacitinib had a manageable tolerability profile in ulcerative colitis patients that was consistent with that established in rheumatoid arthritis patients and longer-term safety data revealed no new safety signals.

Similar content being viewed by others
References
Antonelli E, Torti G, Bassotti G. Inhibitors of the Janus kinases: a new oral treatment option for ulcerative colitis. J Clin Gastroenterol. 2019;53(9):635–40.
Danese S, Grisham M, Hodge J, et al. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310(3):G155–62.
Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1-106.
Chang S, Hudesman D. First-line biologics or small molecules in inflammatory bowel disease: a practical guide for the clinician. Curr Gastroenterol Rep. 2020;22(2):7.
Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–84.
Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450–61.
Lefevre PLC, Vande CN. Clinical pharmacology of janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2020;11(Suppl 2):S725–36.
Ma C, Battat R, Dulai PS, et al. Innovations in oral therapies for inflammatory bowel disease. Drugs. 2019;79(12):1321–35.
Zundler S, Neurath MF. Integrating immunologic signaling networks: the JAK/STAT pathway in colitis and colitis-associated cancer. Vaccines. 2016;4(1):5.
Xeljanz (tofacitinib) 5 and 10 mg film-coated tablets/Xeljanz (tofacitinib) 11 mg prolonged-release tablets: EU summary of product characteristics. Bruxelles: Pfizer Europe MA EEIG; 2020.
Xeljanz (tofacitinib) 5 and 10 mg tablets, for oral use; Xeljanz XR (tofacitinib) 11 and 22 mg extended-release tablets, for oral use: US prescribing information. New York: US Pharmaceuticals, Divison of Pfizer Inc; 2020.
Sayoc-Becerra A, Krishnan M, Fan S, et al. The JAK-inhibitor tofacitinib rescues human intestinal epithelial cells and colonoids from cytokine-induced barrier dysfunction. Inflamm Bowel Dis. 2020;26(3):407–22.
Cordes F, Lenker E, Spille LJ, et al. Tofacitinib reprograms human monocytes of IBD patients and healthy controls toward a more regulatory phenotype. Inflamm Bowel Dis. 2020;26(3):391–406.
Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36.
Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–24.
Rubin D, Dubinsky M, Danese S, et al. Efficacy and safety of an additional 8 weeks of tofacitinib induction therapy: updated results of the OCTAVE Open study for tofacitinib 8-week induction non-responders [poster no. P653]. In: 15th Congress of the European Crohn's and Colitis Organisation (ECCO). 2020.
Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51(2):271–80.
Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(1):139–47.
Travis S, Dotan I, Danese S, et al. Impact of disease duration on tofacitinib efficacy in patients with ulcerative colitis [abstract no. P334]. J Crohns Colitis. 2020;14(Suppl 1):S321–3.
Lichtenstein GR, Cohen BL, Salese L, et al. Impact of baseline corticosteroid therapy on tofacitinib induction efficacy and infection risk in patients with ulcerative colitis: data from global clinical trials [abstract no. P0514 + poster]. Am J Gastroenterol. 2019;114(Suppl):S415.
Farraye FA, Qazi T, Kotze PG, et al. Analysis of the impact of body mass index on efficacy and safety in the tofacitinib OCTAVE ulcerative colitis program. Gastroenterology. 2019;156(6 Suppl 1):S1111–2.
Hanauer S, Rubin DT, Gionchetti P, et al. Tofacitinib efficacy in patients with moderate to severe ulcerative colitis: subgroup analyses of OCTAVE Induction 1 and 2 and OCTAVE Sustain by 5-aminosalicylates use [abstract no. P2321 + poster]. J Crohns Colitis. 2019;13(Suppl 1):S477.
Dubinsky MC, Hudesman DP, Steinwurz F, et al. CRP levels and PMS as early predictors of clinical and endoscopic outcomes in adult patients with moderately-to-severely active UC treated with tofacitinib: a post hoc analysis of OCTAVE Induction 1 and 2 [abstract no. P366]. J Crohns Colitis. 2020;14(Suppl 1):S346–7.
Panes J, Vermeire S, Lindsay JO, et al. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohns Colitis. 2018;12(2):145–56.
Reguerio M, Kinnucan JA, Ungaro R, et al. Predictors of delayed response to tofacitinib: results of the OCTAVE Open study for delayed responders [Poster no. 2283]. In: American College of Gasterenterology Annual Scientific Meeting. 2019.
Dubinsky MC, Chou J-W, Su C, et al. Time to loss of efficacy among patients in remission following induction treatment with tofacitinib 10 mg BID who reduced tofacitinib dose or discontinued tofacitinib in OCTAVE Sustain [presentation no. DOP81]. In: 15th Congress of the European Crohn's and Colitis Organisation (ECCO). 2020.
Dubinsky MC, Clarke K, Klaus JG, et al. Time to loss of efficacy following tofacitinib interruption in patients with ulcerative colitis: results from OCTAVE Sustain [abstract no. OP305 + slide presentation]. United Eur Gastroenterol J. 2018;6(8 Suppl):A121.
Reinisch W, Osterman MT, Doherty G, et al. Efficacy of tofacitinib maintenance therapy for ulcerative colitis in remitting patients vs. patients with clinical response after 8 weeks of induction treatment [abstract no. Tu 1720 + poster]. J Crohns Colitis. 2019;13(Suppl 1):S378–9.
Sands BE, Moss AC, Armuzzi A, et al. Efficacy and safety of escalation to tofacitinib 10 mg BID for patients with UC following loss of response on 5 mg BID maintenance therapy: results from OCTAVE open-label, long-term extension study [abstract no. DOP73]. J Crohns Colitis. 2020;14(Suppl 1):S110–1.
Rubin DT, Dubinsky MC, Panés J, et al. Efficacy of tofacitinib dose escalation to 10 mg BID in patients with UC after completing maintenance therapy in remission and losing response: data from OCTAVE open-label, long-term extension study [abstract no. DOP75]. J Crohns Colitis. 2020;14(Suppl 1):S112–3.
Colombel JF, Osterman MT, Ibanez P, et al. Maintenance of remission with tofacitinib in patients with ulcerative colitis: updated results of a subpopulation analysis from an open-label, long-term extension study, OCTAVE Open [abstract no. DOP59]. J Crohns Colitis. 2020;14(Suppl 1):S098-S99.
Chiorean M, Su C, Matsuoka K, et al. Efficacy and safety of open-label treatment with tofacitinib 10 mg twice daily in patients with ulcerative colitis with clinical response, but not remission, after 52 weeks of maintenance therapy: data from the OCTAVE studies [abstract no. DOP41]. J Crohns Colitis. 2019;13(Suppl 1):S050.
Rudrapatna VA, Butte AJ. Mind the gap: a comparison of the randomized clinical trial efficacy and real world effectiveness of tofacitinib for the treatment of inflammatory bowel disease [abstract no. 861]. Am J Gastroenterol. 2019;114(Suppl):S499-500.
Lair-Mehiri L, Stefanescu C, Vaysse T, et al. Real-world tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. J Crohns Colitis. 2019;13(Suppl 1):S478–9.
Ungaro R, Fenster M, Dimopoulos C, et al. Real-world effectiveness of tofacitinib in ulcerative colitis: a multi-centre study [abstract no. P344]. J Crohns Colitis. 2019;13(Suppl 1):S274–5.
Clark-Snustad KD, Singla A, Lee SD. Tofacitinib improves clinical disease activity in a real-world population of patients with moderate-severe ulcerative colitis and Crohn’s disease [abstract no. Su1928]. Gastroenterology. 2019;156(6 Suppl 1):S664.
Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. Real-world experience with tofacitinib in IBD at a tertiary center. Dig Dis Sci. 2019;64(7):1945–51.
Long M, Abdalla M, McCabe R, et al. Methodology and initial results from TOUR (tofacitinib response in ulcerative colitis): a real world prospective multicenter study [abstract no. P056]. Am J Gastroenterol. 2019;114(Suppl):S15.
Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multi-centre UK experience. J Crohns Colitis. 2020;14(10):1385–93.
Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–8.
Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2020;14(Suppl 1):S026-S28.
Sandborn WJ, Panes J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17(8):1541–50.
Sandborn WJ, Panes J, Panaccione R, et al. Tofacitinib for the treatment of ulcerative colitis: up to 5.4 years of safety: data from global clinical trials [abstract no. Tu1717 + poster]. J Crohns Colitis. 2019;13(Suppl 1):S344.
Lichtenstein GR, Moore GT, Soonasra A, et al. Analysis of haematological changes in tofacitinib-treated patients with ulcerative colitis across phase 3 induction and maintenance studies [abstract no. Tu1752 + poster]. J Crohns Colitis. 2019;13(Suppl 1):S351–2.
Sandborn WJ, Panes J, BE S, et al. Incidence of venous thromboembolic events in patients with ulcerative colitis treated with tofacitinib in the ulcerative colitis clinical programme: an update as of May 2019 [poster no. P598]. In: 15th Congress of the European Crohn's and Colitis Organisation (ECCO). 2020.
Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(1):123-32.e3.
Winthrop KL, Loftus EV, Baumgart DC, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of infection rates in the tofacitinib ulcerative colitis clinical program [abstract no. P1371 + poster]. Am J Gastroenterol. 2019;114(Suppl):S427–9.
Lichtenstein GR, Loftus EV Jr, Wei S-C, et al. Tofacitinib, an oral, small molecule janus kinase inhibitor, in the treatment of ulcerative colitis: analysis of an open-label, long-term extension study with up to 5.9 years of treatment [abstract no. Tu1857]. Gastroenterology. 2020;158(6 Suppl 1):S1190–1.
Lichtenstein GR, Bressler B, Vermeire S, et al. Assessment of age as a risk factor for adverse events in patients from the tofacitinib ulcerative colitis clinical program [abstract no. Tu1858]. Gastroenterology. 2020;158(6 Suppl 1):S1191.
Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24(10):2258–65.
Trigo-Vicente C, Gimeno-Ballester V, Garcia-Lopez S, et al. Systematic review and network meta-analysis of treatment for moderate-to-severe ulcerative colitis. Int J Clin Pharm. 2018;40(6):1411–9.
Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18(10):2179–2191.e6.
Pantavou K, Yiallourou AI, Piovani D, et al. Efficacy and safety of biologic agents and tofacitinib in moderate-to-severe ulcerative colitis: a systematic overview of meta-analyses. United Eur Gastroenterol J. 2019;7(10):1285–303.
Bonovas S, Lytras T, Nikolopoulos G, et al. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(4):454–65.
Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(2):162–75.
Quon P, Sardesai A, Milev S, et al. Cost-effectiveness of tofacitinib compared with infliximab, adalimumab, golimumab and vedolizumab for the treatment of moderate to severe ulcerative colitis in Germany [abstract no. PGI9]. Value Health. 2019;22(Suppl 3):S617.
Vellopoulou K, Stefanou G, Tzanetakos C, et al. Cost-effectiveness analysis of tofacitinib for the treatment of moderate to severe active ulcerative colitis in Greece [abstract no. PGI19]. Value Health. 2019;22(Suppl 3):S619.
Diamantopoulos A, Lohan C, LeReun C, et al. An economic evaluation of tofacitinib in the treatment of moderately to severely active ulcerative colitis in the United Kingdom [abstract no. PGI29]. Value Health. 2019;22(Suppl 3):S621.
Lohan C, Diamantopoulos A, LeReun C, et al. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol. 2019;6(1):e000302.
Menchen L, de Andres-Nogales F, Garcia S, et al. Cost-effectiveness of tofacitinib for the treatment of moderate-to-severe ulcerative colitis after biologic failure or intolerance in Spain [abstract no. PGI16]. Value Health. 2019;22(Suppl 3):S619.
Taxonera C, de Andres-Nogales F, Garcia S, et al. Cost-effectiveness of tofactinib for the treatment of moderate-to-severe ulcerative colitis after conventional therapy in Spain [abstract no. PGI17]. Value Health. 2019;22(Suppl 3):S619.
Milev S, DiBonaventura MD, Quon P, et al. An economic evaluation of tofacitinib for the treatment of moderately-to-severely active ulcerative colitis: modeling the cost of treatment strategies in the United States. J Med Econ. 2019;22(9):859–68.
Acknowledgements
The manuscript was reviewed by: R. Caviglia, Gastroenterology and Digestive Endoscopy Department, Barts Health NHS Trust, London, United Kingdom; S. Gayam, Gastroenterology and Hepatology, West Virginia University Medicine, Morgantown, WV, USA; L. Guidi, IBD unit, Division of Gastroenterology, Fondazione Policlinico Universitario A. Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy. During the peer review process, Pfizer, the marketing-authorization holder of tofacitinib, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Y.-A.H. is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Conset to publish, Availability of data and material, Code avaiability
Not applicable.
Additional information
Enhanced material for this Adis Drug Q&A can be found at https://doi.org/10.6084/m9.figshare.12923972.
Rights and permissions
About this article
Cite this article
Heo, YA. Tofacitinib in ulcerative colitis: a profile of its use. Drugs Ther Perspect 36, 553–563 (2020). https://doi.org/10.1007/s40267-020-00789-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-020-00789-9