Abstract
Background and Objective
Prescribing cascades occur when a drug is prescribed to manage side effects of another drug, typically when a side effect is misinterpreted as a new condition. A consensus list of clinically important prescribing cascades that adversely affect older persons’ health (i.e., where risks of the prescribing cascade usually exceed benefits) was developed to help identify, prevent, and manage prescribing cascades.
Methods
Three rounds of a modified Delphi process were conducted with a multidisciplinary panel of 38 clinicians from six countries with expertise in geriatric pharmacotherapy. The clinical importance of 139 prescribing cascades was assessed in Round 1. Cascades highly rated by ≥ 70% of panelists were included in subsequent rounds. Factors influencing ratings in Rounds 1 and 3 were categorized. After three Delphi rounds, highly rated prescribing cascades were reviewed by the study team to determine the final list of clinically important cascades consistent with potentially inappropriate prescribing.
Results
After three rounds, 13 prescribing cascades were highly rated by panelists. Following a study team review, the final tool includes nine clinically important prescribing cascades consistent with potentially inappropriate prescribing. Panelists reported that their ratings were influenced by many factors (e.g., how commonly they encountered the medications involved and the cascade itself, the severity of side effects, availability of alternatives). The relative importance of these factors in determining clinical importance varied by panelist.
Conclusions
A nine-item consensus-based list of clinically important prescribing cascades, representing potentially inappropriate prescribing, was developed. Panelists’ decisions about what constituted a clinically important prescribing cascade were multi-factorial. This tool not only raises awareness about these cascades but will also help clinicians recognize these and other important prescribing cascades. This list contributes to the prevention and management of polypharmacy and medication-related harm in older people.
Similar content being viewed by others
Prescribing cascades are under-recognized contributors to polypharmacy, inappropriate prescribing, and medication-related harm; tools are needed to help prescribers identify these cascades. |
A modified Delphi process with an international multidisciplinary expert panel was used to develop a tool, ThinkCascades, which provides a short list of nine clinically important prescribing cascades affecting older people. |
ThinkCascades raises awareness about these nine prescribing cascades, and the phenomenon of prescribing cascades more broadly. |
1 Introduction
Prescribing cascades are under-recognized contributors to polypharmacy, making them challenging targets for deprescribing. They occur when one drug is prescribed to manage the side effect of another drug, typically when the side effect is misinterpreted as a new medical condition [1,2,3].
Prescribing cascades can result in inappropriate prescribing of new pharmacotherapies, putting people at unnecessary risk for adverse drug events (ADEs) [4,5,6,7], increased pill burden [8], and reduced quality of life [9], as well as additional costs to individuals and healthcare systems [10]. This issue is of particular concern in older persons with multimorbidity and polypharmacy who are more susceptible to ADEs [8]. Preventing, identifying, and managing prescribing cascades is critical and integral to enhancing medication safety [10].
Many clinicians struggle to identify prescribing cascades both conceptually and in clinical practice [11, 12]. The large number of potential prescribing cascades makes them challenging to recognize in practice [10, 13]. Reconstructing the chronology of prescribing is also challenging, especially for patients experiencing transitions in care. Furthermore, when multiple prescribers are involved, responsibility for appropriate prescribing and patient education may also be unclear [11].
Systematic reviews have identified more than 70 explicit tools (criterion based; e.g., STOPP [Screening Tool of Older Persons’ Prescriptions] [14], American Geriatric Society’s Beers criteria [15]) and implicit (judgment based; e.g., Medication Appropriateness Index [16, 17]) to help clinicians prevent, identify, and manage potentially inappropriate medication use [18, 19]. The vast number of tools has arisen because prescribing habits and locally available medications vary considerably between countries, thus guides tailored to the needs of specific regions may be required [20]. With this diversity of tools in such a complex field, it is not possible to distinguish a single ideal tool, rather, clinicians and researchers can select what works best based on the context in which the tool is intended to be used.
Some tools for assessing the quality of prescribing include prescribing cascades [15, 21]. However, there are no explicit tools specifically designed for identifying prescribing cascades. In this study, the iKASCADE consortium used published evidence and expert consensus to create a list of internationally recognized, clinically important prescribing cascades affecting older people [22].
2 Methods
2.1 Research Steering Committee
The iKASCADE Consortium, a multidisciplinary group of international experts in geriatric pharmacotherapy, planned and conducted this study [22]. Consortium members (herein referred to as the ‘study team’) did not participate as panelists in the Delphi process.
2.2 Study Design
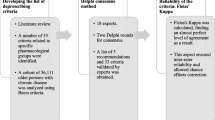
We conducted a multi-step consensus development project (Fig. 1). To create an inventory of prescribing cascades, we completed a literature review and iteratively refined the inventory through discussions with the study team. We were intentionally inclusive when creating the inventory, i.e., included cascades that could be intentional (the side effect was recognized as such) or unintentional (the side effect may not have been recognized as related to Drug A) [3]. We then used a modified Delphi process to obtain expert consensus on the clinical importance of the identified prescribing cascades affecting older people. Finally, the study team reviewed the results of the Delphi process to confirm a final list of clinically important cascades most consistent with potentially inappropriate prescribing for older adults.
Our approach was based on the development processes of the STOPP/START criteria [23, 24], and deprescribing clinical practice guidelines [25]. Study methods and reporting were guided by the CREDES Guidance on Conducting and Reporting Delphi Studies [26]. The Research Ethics Board at Women’s College Hospital approved this study (2019-0188-E).
2.2.1 Phase I: Delphi Planning
2.2.1.1 Selection and Identification of the Delphi Panel
Study team members provided a rank-ordered list of nominees from their country for the expert panel. Eligibility criteria included registered health professionals with expertise in pharmacotherapy for older people who were fluent in English. “Expertise” was defined as one or more of the following: (1) 4 or more years of experience providing care for older people; (2) academic appointments involving a geriatric focus; (3) academic publications in the geriatrics field; and/or (4) training or certification in the care of older persons. We recruited geriatricians, primary care physicians, pharmacists, and nurses meeting these expertise criteria. Nurses from outside North America and pharmacists from Italy were excluded as they are not usually closely involved in prescribing or managing pharmacotherapy.
2.2.1.2 Recruitment and Panel Size
The study coordinator e-mailed nominees, based on rank order, until two panelists from each professional group in each country consented to participate in Round 1 of the Delphi process. We aimed to recruit ≥ 38 panelists; two people from each of four professional groups in Canada and the USA; two people from each of three professional groups in Belgium, Ireland, and Israel (geriatricians, primary care physicians, pharmacists), and two people from the two professional groups (geriatricians, family physicians) in Italy.
2.2.1.3 Compiling an Inventory of Prescribing Cascades
An inventory of ‘potential’ prescribing cascades was compiled between January and October 2019 using a four-step process (for details, eAppendix 1 in the Electronic Supplementary Material [ESM]). In the inventory, Drug A represents the first medication prescribed, which leads to a side effect that results in prescribing of another medication, Drug B. Examples of prescribing cascades were extracted from the literature known to the study team [10, 27]. A search strategy from a previous scoping review [10] was re-executed to identify additional sources (databases: MEDLINE, EMBASE, CINAHL, PsycINFO, Cochrane Library [inception to February 2019], total 318 citations). Clinical examples not captured in the literature review were also gathered during study team meetings. Study team members reviewed the draft inventory in two phases, suggested additional prescribing cascades, and approved the final list of 139 unique prescribing cascades included in Round 1 (eTable 1a–1i of the ESM).
2.2.1.4 Defining a Clinically Important Prescribing Cascade
A clinically important prescribing cascade was defined as one in which the risks of prescribing Drug A and B together likely exceed the benefits of the combination (Fig. 2). We were interested in how a diverse group of panelists rated prescribing cascades; consequently, we kept our definition broad and did not use terms like problematic, inappropriate, or appropriate.
To assist panelists, we provided considerations regarding clinical importance to reflect upon as they rated cascades (Fig. 2). These considerations were originally developed by our investigators; two additional criteria were added for Round 3 based on panelist comments from Round 1.
2.2.2 Phase II: Modified Delphi Surveys: Administration and Analysis
Three rounds of online surveys were administered between March 2020 and March 2021 with interruptions in data collection because of the extenuating circumstances of the coronavirus disease 2019 (COVID-19) pandemic. The results of each round were reviewed by the study team for consistency and data quality.
2.2.2.1 Round One
Participant demographics including profession (physician [primary care, geriatrician], pharmacist, nurse), years of practice experience, practice setting, and country were gathered. Participants were asked to share their sex at birth and gender identity. Then, the list of 139 prescribing cascades were presented to panelists, grouped by physiologic system of Drug A. Panelists were asked to rate the clinical importance of all 139 prescribing cascades affecting older people on a 5-point Likert scale (1 = ‘definitely not important’; 5 = ‘definitely important’) with an option to indicate when they were ‘not sure’. Free-text comment boxes invited participants to comment on why each cascade was or was not clinically important.
Free-text responses were analyzed using an inductive content analysis approach [28] completed by two study team members; themes were added to the list of considerations shared with all panelists in Round 3 (Fig. 2). Prescribing cascades rated as probably (‘4’) or definitely important (‘5’) by ≥ 70% of panelists [25] were retained for Round 2. Participants were invited to provide additional examples of prescribing cascades that were missing from the survey; any examples provided by > 10% of panelists were included in Round 2.
2.2.2.2 Round Two
Panelists were presented with the rating for each prescribing cascade rated as ‘4’ or ‘5’ by ≥ 70% of panelists in Round 1 and a histogram showing their ratings compared to other panelists (eAppendix 2 of the ESM). Respondents were then asked to rank these cascades in order of importance. Because of the constraints of the survey platform, cascades were presented in alphabetical order. Kendall’s W coefficient of concordance (W), which measures associations amongst ranked data, was calculated for the overall group, within healthcare professional groups, and by country, to determine whether consensus (W = 0.7) had been achieved and to determine whether a subsequent round of surveys was necessary [25].
2.2.2.3 Round Three
To address challenges associated with ranking the large number of items, including response order bias, we adjusted the study protocol to focus on achieving consensus ratings of clinical importance (versus consensus rankings). Within the survey platform, panelists were shown prescribing cascades rated as ‘4’ or ‘5’ in Round 1, the histograms comparing their rating with other panelists (eAppendix 2 of the ESM) and were asked to rate the clinical importance of each prescribing cascade using the same 5-point Likert scale (including the ‘not sure’ option). To minimize response order bias, three versions of the survey were created, varying the cascades’ presentation order. An online randomization system was used to assign panelists to one of the three survey versions. At the end of the survey, panelists were asked to share global reflections about factors that guided their ratings of clinical importance, including whether some factors had guided ratings more than others.
Prescribing cascades rated as probably (‘4’) or definitely important (‘5’) by ≥ 70% of panelists were retained for Phase III. Open-ended responses were analyzed using an inductive content analysis approach [28] involving two study team members.
2.2.3 Phase III: ThinkCascades Tool Creation
Rather than reporting Delphi panel results as the final list of clinically important prescribing cascades, the study team felt it essential that for the ThinkCascades to be a useful tool for clinicians, it must focus on prescribing cascades that represent examples of potentially inappropriate prescribing. To us, examples of cascades representing potentially inappropriate prescribing typically occur when a clinician misinterprets a side effect as a new medical condition and prescribes a second drug. Accordingly, though not specified as a step in our original study protocol, examples less aligned with the study definition of clinical importance and not clearly consistent with potentially inappropriate prescribing were omitted from the ThinkCascades tool.
3 Results
3.1 Round One
Of 111 invited participants, 54 consented and 40 completed Round 1. The panel included geriatricians, primary care physicians, pharmacists, and nurses from six countries; consisting of 23 women and 17 men (all of whom were cis-gendered) of which 33 had ≥ 10 years of clinical practice experience (Table 1; eTable 2 of the ESM displays profession and country by sex). Continued involvement of participants through the study phases is shown in eFigure 1 of the ESM.
As shown in Table 2, 32 of 139 prescribing cascades from the inventory achieved a rating of probably (‘4’) or definitely important (‘5’) by ≥ 70% of the panelists in Round 1. No additional prescribing cascade examples were added by panelists. On review of results from Round 1, the study team identified two prescribing cascades that could be consolidated.
When asked about factors considered when rating prescribing cascades, respondents provided additional considerations. These included: the indication and appropriateness of prescribing drug A, and how commonly drug A is prescribed. These factors were added to the list of considerations shared with all panelists in Round 3 (Fig. 2).
3.2 Round Two
All 40 panelists who completed Round 1 were invited to complete Round 2; 35 panelists did so (88% response rate) (Table 1). Kendall’s W coefficient of concordance across all 35 respondents was 0.17, lower than our predetermined threshold for consensus (0.70). Kendall’s W within professions and by country are presented in eTable 3 of the ESM. When reviewing Round 2 results, the study team observed a pattern of responses in the data, consistent with response order bias (i.e., that the cascades that appeared “lower” in the list were rated lower, see eAppendix 2 of the ESM).
3.3 Round Three
All 35 panelists completing Round 2 were invited to complete Round 3; 31 panelists did so (89% response rate) (Table 1). As shown in Table 2, 13 prescribing cascades were rated as ‘4’ or ‘5’ by ≥ 70% of panelists. Ratings from each prescribing cascade by sex, gender, country, and profession are presented in eTable 4 of the ESM. Consistent rating patterns were observed between female and male clinicians for prescribing cascades in the cardiovascular and musculoskeletal physiologic systems; discordant ratings were observed for cascades within the central nervous and urogenital systems, and the anti-infective prescribing cascade involving Clostridioides difficile.
Country-specific differences between panelists were observed, but small sample sizes precluded conclusions about patterns. Nearly two-thirds (21/31) of Round 3 panelists represented geriatricians and pharmacists; ratings for 10 of 12 prescribing cascades were quite consistent across these professional groups (eTable 4 of the ESM).
Twenty-one respondents answered the open-ended question about which factors were most important for guiding their ratings. Panelists indicated that the severity of the side effect caused by drug A, and the ability of clinicians to anticipate and manage the side effect without prescribing drug B, were key factors influencing their ratings.
3.4 Phase III: ThinkCascades Tool Creation
During our study team’s review after the Delphi process was complete, four changes were made to the final list of clinically important prescribing cascades representing potentially inappropriate prescribing to develop the ThinkCascades tool. First, we consolidated two similar cascades (urinary anticholinergic and antimuscarinic for overactive bladder prescribing cascades, both of which were highly rated by Delphi panelists) to avoid duplication. Second, after we carefully reviewed the Delphi results for alignment with the study definition of clinical importance (Fig. 2) and to ensure the ThinkCascades tool features examples of potentially inappropriate prescribing, the study team removed three cascades. The exclusions are presented in Table 2 with additional rationale provided in eAppendix 3 of the ESM. After these exclusions, the final short consensus-based ThinkCascades tool includes nine clinically important cascades that are examples of potentially inappropriate prescribing affecting older people (Table 3).
4 Discussion
We developed an expert consensus-based tool consisting of a short list of clinically important prescribing cascades affecting older people that are examples of potentially inappropriate prescribing. Our study was rigorously conducted and represents the views of a diverse international and multidisciplinary panel of expert clinicians who are routinely involved in pharmacotherapy management for older persons. ThinkCascades, as a tool that lists clinically important prescribing cascades consistent with potentially inappropriate prescribing, is valuable as it increases awareness of an important aspect of potentially inappropriate prescribing. Our study highlights a select group of clinically important prescribing cascades that may also help clinicians to recognize and become attuned to other important prescribing cascades and avoidable medication-related harm for older people.
ThinkCascades is not intended to be a comprehensive list of all clinically important prescribing cascades. Instead, it is a short list of highly rated prescribing cascades incorporating diverse views across professions and countries focused on showcasing examples of potentially inappropriate prescribing for older adults. Clinicians across care settings can use this list as one of the tools for guiding decisions about appropriate pharmacotherapy for their older patients. Furthermore, this tool may prompt clinicians to recognize other prescribing cascades during a medication review. As with other instruments for identifying sources of potentially inappropriate prescribing, this short consensus list of clinically important prescribing cascades is not intended to replace clinical judgment. When medications are intentionally prescribed after a deliberation process that actively incorporates patient circumstances, values and preferences, best available evidence, and therapeutic alternatives, prescribers might reasonably opt to initiate or continue a prescribing cascade that appears on this list.
When developing the inventory of prescribing cascades for rating by panelists, we intentionally kept our definitions broad. Consistent with best practice guidance for Delphi studies, we aimed to avoid introducing our own perceptions which might directly or indirectly impact panelists’ judgments [26]. As such, we avoided ‘filtering’ the inventory a priori, i.e., we did not attempt to predetermine which examples were most clinically important or considered appropriate versus inappropriate.
When the Delphi panel results were reviewed by our study team, there was consensus that three cascades were different from the others. These examples describe prescribing cascades where the combination of Drug A and Drug B was likely needed and beneficial, i.e., the risks of the combination did not outweigh the benefits. For example, two were considered examples of usually intentional and appropriate prescribing cascades (i.e., non-steroidal anti-inflammatory drugs → gastritis/gastric ulcer/gastrointestinal bleed → gastroprotective agent [proton pump inhibitor, histamine H2-receptor antagonist]; and diuretic → hypokalemia → potassium supplement) [3]. One could speculate that these cascades were highly rated because panelists gave greater consideration to the appropriateness of Drug A for the underlying condition, rather than applying an overall focus on the risk-benefit ratio. As such, these cascades were removed from the final list. In addition, we were concerned about confusion, and possible patient harm, that might occur if the three usually intentional and often appropriate cascades were inadvertently interpreted by users of the tool as examples of potentially inappropriate prescribing for older adults.
Consistent with best practice, our study team did not participate as panelists in the Delphi process; instead, we nominated experienced clinicians who participated as panelists despite competing demands from the COVID-19 pandemic. Alternatives to the study team selecting examples most consistent with potentially inappropriate prescribing may have been to convene an additional Delphi round following a discussion with panelists. This was impractical because of the ongoing COVID-19 pandemic but raises opportunities for future research.
Our study identified variability in panelists’ responses by profession and country of origin. We observed some consistency in ratings among geriatricians and pharmacists but less among general practitioners and nurses. This variability suggests that decision-making processes for determining clinically important prescribing cascades are multifactorial [11]. Differences observed in ratings for some cascades based on panelists’ sex and country are interesting yet merit further investigation and confirmation in studies designed to explore potential influences of gender and other factors on clinicians’ ratings. Further, these observations raise opportunities for future research exploring how clinician characteristics impact approaches to prescribing broadly, as well as prescribing cascades.
Ratings of importance were influenced by how commonly panelists encountered a particular cascade in their own practice, how serious they perceived the ‘side effect’ to be, and whether there were non-pharmacological or ‘less risky’ drug alternatives that could be used in place of Drug A. Consistent with qualitative research about cascades, these findings suggest that there are many factors that contribute to clinicians’ ratings of clinically important prescribing cascades [11]. Future studies to elucidate what constitutes a clinically important prescribing cascade and the factors that clinicians prioritize in this deliberation are needed. Furthermore, interventions to help clinicians both increase their awareness of prescribing cascades and their ability to identify, prevent, and manage them in practice are important areas for future investigation.
This study has some limitations. First, this modified Delphi process with an international and multidisciplinary panel of clinicians was undertaken during the COVID-19 pandemic with lapses in data collection because of extenuating circumstances in different countries. Nevertheless, we achieved a nearly 90% response rate in all three rounds. Second, we did not achieve consensus in Round 2 (ranking) and response order bias was observed. To address this, in Round 3, we asked panelists to rate rather than rank importance and randomized the order in which prescribing cascades were presented. Third, despite high response rates in all rounds, during data collection for Round 3, panelists expressed difficulty with continuing to participate because of clinical demands from the pandemic, despite their commitment to the study. This led us not to confirm their agreement with exclusions made on the final ThinkCascades tool.
5 Conclusions
We developed an expert consensus-based short list of highly rated, clinically important prescribing cascades impacting older people that may represent potentially inappropriate prescribing using an international modified Delphi process. Our panel consisted of a diverse group of experienced clinicians from six countries and four professions with expertise in pharmacotherapy management for older people. Our findings highlight that clinicians’ decisions about whether a prescribing cascade is clinically important are multifactorial. ThinkCascades provides nine practical prescribing cascades that clinicians should consider when prescribing and reviewing medications across care settings, countries, and professions. Furthermore, the tool may facilitate clinicians’ identification of other prescribing cascades in their practices.
References
Rochon PA, Gurwitz JH. Drug therapy. Lancet. 1995;346(8966):32–6.
Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096–9.
McCarthy LM, Visentin JD, Rochon PA. Assessing the scope and appropriateness of prescribing cascades. J Am Geriatr Soc. 2019;67(5):1023–6.
Savage RD, Visentin JD, Bronskill SE, Wang X, Gruneir A, Giannakeas V, et al. Evaluation of a common prescribing cascade of calcium channel blockers and diuretics in older adults with hypertension. JAMA Intern Med. 2020;180(5):643–51.
Read SH, Giannakeas V, Pop P, Bronskill SE, Herrmann N, Chen S, et al. Evidence of a gabapentinoid and diuretic prescribing cascade among older adults with lower back pain. J Am Geriatr Soc. 2021;69(10):2842–50.
Lega IC, Bronskill SE, Campitelli MA, Guan J, Stall NM, Lam K, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21(11):2394–404.
Gill SS, Mamdani M, Naglie G, Streiner DL, Bronskill SE, Kopp A, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–13.
Rochon PA, Gurwitz JH. The prescribing cascade revisited. Lancet. 2017;389(10081):1778–80.
Morris EJ, Brown JD, Manini TM, Vouri SM. Differences in health-related quality of life among adults with a potential dihydropyridine calcium channel blocker-loop diuretic prescribing cascade. Drugs Aging. 2021;38(7):625–32.
Brath H, Mehta N, Savage RD, Gill SS, Wu W, Bronskill SE, et al. What is known about preventing, detecting, and reversing prescribing cascades: a scoping review. J Am Geriatr Soc. 2018;66(11):2079–85.
Farrell BJ, Jeffs L, Irving H, McCarthy LM. Patient and provider perspectives on the development and resolution of prescribing cascades: a qualitative study. BMC Geriatr. 2020;20(1):368.
Piggott KL, Mehta N, Wong CL, Rochon PA. Using a clinical process map to identify prescribing cascades in your patient. BMJ. 2020;368: m261.
Morris EJ, Hollmann J, Hofer A-K, Bhagwandass H, Oueini R, Adkins LE, et al. Evaluating the use of prescription sequence symmetry analysis as a pharmacovigilance tool: a scoping review. Res Social Adm Pharm. 2022;18(7):3079–93.
O’Mahony D. STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: origin and progress. Exp Rev Clin Pharmacol. 2020;13(1):15–22.
American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94.
Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–51.
Somers A, Mallet L, van der Cammen T, Robays H, Petrovic M. Applicability of an adapted medication appropriateness index for detection of drug-related problems in geriatric inpatients. Am J Geriatr Pharmacother. 2012;10(2):101–9.
Kaufmann CP, Tremp R, Hersberger KE, Lampert ML. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol. 2014;70(1):1–11.
Pazan F, Kather J, Wehling M. A systematic review and novel classification of listing tools to improve medication in older people. Eur J Clin Pharmacol. 2019;75(5):619–25.
Petrovic M, Gnjidic D, Tommelein E, Boussery K. Pharmacotherapy. In: Roller-Wirnsberger R, Singler K, Polidori MC, editors. Learning geriatric medicine: a study guide for medical students. Cham: Springer International Publishing; 2018. p. 219–35.
Rochon PA, Petrovic M, Cherubini A, Onder G, O’Mahony D, Sternberg SA, et al. Polypharmacy, inappropriate prescribing, and deprescribing in older people: through a sex and gender lens. Lancet Healthy Longevity. 2021;2(5):e290-300.
Sternberg SA, Petrovic M, Onder G, Cherubini A, O’Mahony D, Gurwitz JH, et al. Identifying key prescribing cascades in older people (iKASCADE): a transnational initiative on drug safety through a sex and gender lens-rationale and design. Eur Geriatr Med. 2021;12(3):475–83.
Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J, Pottie K. What are priorities for deprescribing for elderly patients? Capturing the voice of practitioners: a modified Delphi process. PLoS ONE. 2015;10(4): e0122246.
Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med. 2017;31(8):684–706.
Rochon PA, Gurwitz JH. Supplement to: the prescribing cascade revisited. Lancet. 2017;389(10081):1778–80.
Schreier M. Qualitative content analysis. In: Flick U, editor. The SAGE handbook of qualitative data analysis. London: SAGE Publications Ltd; 2014. Available from: https://methods.sagepub.com/book/the-sage-handbook-of-qualitative-data-analysis. Accessed 24 Aug 2021.
Acknowledgements
We acknowledge Jennifer Ackerman and Peter Anderson from the iKASCADE team for their contributions to the study. We thank ZhiDi Deng, Dana Green-Campbell, Romina Isip, Dr. Miles Luke, Sanket Patel, and Shawn Varghese for their assistance in building the inventory of prescribing cascades that informed Round 1 and Drs. Nathan Stall and Kenneth Lam for their clinical review of the inventory. Last, we thank all of our panelists. The names of the panelists who consented to be listed appear in eAppendix 4 of the ESM. Dr. McCarthy was a member of the Junior Investigator Intensive Program of the US Deprescribing Research Network, which is funded by the National Institute on Aging (R24AG064025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The iKASCADE project is funded by “Identifying Key Prescribing CASCADes in the Elderly: A Transnational Initiative on Drug Safety” (GNP-1782), GENDER-NET Plus ERA-Net Cofund: “Promoting gender equality in H2020 and the ERA”, in Partnership with: the Canadian Institutes of Health Research-Institutes of Gender & Health and Institute of Aging; the Irish Research Council; Ministerodella Salute (Italy); and Ministry of Science and Technology (Israel). Dr. Paula Rochon holds the RTOERO Chair in Geriatric Medicine at the University of Toronto. The sponsor was not involved in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this study.
Ethics approval
The Research Ethics Board at Women’s College Hospital approved this study (2019-0188-E).
Consent to Participate
All panelists consented to participate. Panelists listed in the Supplementary material (eAppendix 4 of the ESM) consented to publication of their names and jurisdictions.
Consent for Publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Author contributions
PAR and LMM have full access to all study data; LMM takes responsibility for the integrity of the data and accuracy of the data analyses. Concept and design: PAR, LMM, RS, RM, AL, JL, WW, and the iKASCADE team. Acquisition, analysis or interpretation of the data: all authors. Drafting of the manuscript: LMM, RS, KD, JL, AL, RM, and PAR. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: WW. Administrative, technical or material support: LMM, JL, AL, and PAR. Supervision: LMM and PAR.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCarthy, L.M., Savage, R., Dalton, K. et al. ThinkCascades: A Tool for Identifying Clinically Important Prescribing Cascades Affecting Older People. Drugs Aging 39, 829–840 (2022). https://doi.org/10.1007/s40266-022-00964-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-00964-9