Abstract
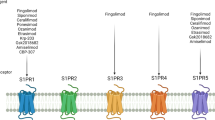
Etrasimod (VELSIPITY™) is an orally available, small-molecule selective sphingosine-1-phosphate (S1P) receptor modulator being developed by Pfizer for the treatment of ulcerative colitis and other immune-mediated inflammatory disorders. Etrasimod is selective for S1P receptor subtypes S1P1, S1P4 and S1P5 while having minimal activity on S1P3 and no activity on S1P2. Etrasimod received its first approval, in the USA, in October 2023 for the treatment of moderately to severely active ulcerative colitis in adults. Subsequently, the European Medicines Agency adopted a positive opinion in December 2023, recommending the granting of marketing authorisation for etrasimod for the treatment of patients aged ≥ 16 years with moderately to severely active ulcerative colitis who have had an inadequate response, lost response, or were intolerant to either conventional therapy, or a biological agent. Etrasimod is also under regulatory review for the treatment of ulcerative colitis in several other countries. Clinical development of etrasimod for use in the treatment of Crohn's disease, atopic dermatitis, eosinophilic oesophagitis and alopecia areata is ongoing worldwide. This article summarises the milestones in the development of etrasimod leading to this first approval for the treatment of ulcerative colitis in adults.
Similar content being viewed by others
References
Buzard DJ, Kim SH, Lopez L, et al. Discovery of APD334: design of a clinical stage functional antagonist of the sphingosine-1-phosphate-1 receptor. ACS Med Chem Lett. 2014;5(12):1313–7.
US FDA. VELSIPITY™ (etrasimod) tablets, for oral use: US prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216956s000lbl.pdf. Accessed 11 Jan 2024.
Mendelson K, Evans T, Hla T. Sphingosine 1-phosphate signalling. Development. 2014;141(1):5–9.
US FDA. VELSIPITY™ (etrasimod) tablets, for oral use: NDA approval. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216956Orig1s000ltr.pdf. Accessed 11 Jan 2024.
Siegmund B, Kiyomi K, Abreu M, et al. Effect of etrasimod on immune cell subsets in colonic tissue of patients with ulcerative colitis: immunophenotyping analysis of colon biopsy samples from the phase 3 ELEVATE UC 52 and ELEVATE UC 12 trials [abstract no. Tu1724 plus poster]. Gastroenterology. 2023;164(6 Suppl):S-1095.
European Medicines Agency. Summary of opinion (initial authorisation) - Velsipity (etrasimod). 2023. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-velsipity_en.pdf. Accessed 11 Jan 2024.
Pfizer. Pfizer completes acquisition of Arena Pharmaceuticals [media release]. 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-completes-acquisition-arena-pharmaceuticals. Accessed 11 Jan 2024.
Everest Medicines. Everest Medicines achieved first patient dosing milestone in etrasimod phase 3 clinical study in ulcerative colitis patients in Asia [media release]. 2019. https://www.everestmedicines.com/news/everest-medicines-announces-completion-of-patient-enrollment-in-phase-3-clinical-trial-of-etrasimod-in/63cf7489-0bdc-415b-8981-a531f9cbf5e2/. Accessed 11 Jan 2024.
Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet. 2023;401(10383):1159–71.
Lee CA, Oh DA, Tang Y, et al. Disposition and mass balance of etrasimod in healthy subjects and in vitro determination of the enzymes responsible for its oxidative metabolism. Clin Pharmacol Drug Dev. 2023;12(6):553–71.
Lee C, Ramaiya A, Tang Y, et al. A phase 1 study evaluating the bioequivalence of the proposed commercial and clinical formulations of etrasimod 2mg, and the effect of food on the pharmacokinetics of the proposed commercial formulation in healthy volunteers [abstract no. OP27]. J Crohns Colitis. 2022;16(Suppl_1):i029.
Lee C, Tang Y, Schroeder C, et al. Pharmacokinetics, safety, and tolerability of etrasimod (APD334) in subjects with mild, moderate, or severe hepatic impairment: a single-dose, open-label, parallel-group study [abstract no. P291]. J Crohns Colitis. 2021;15(Suppl_1):S321–2.
Sandborn WJ, Peyrin-Biroulet L, Zhang J, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):550–61.
Vermeire S, Chiorean M, Panés J, et al. Long-term safety and efficacy of etrasimod for ulcerative colitis: results from the open-label extension of the OASIS study. J Crohns Colitis. 2021;15(6):950–9.
D’Haens G, Dubinsky MC, Peyrin-Biroulet L, et al. Etrasimod induction therapy in moderately to severely active Crohn’s disease: results from a phase 2, randomised, double-blind substudy [abstract no. P632]. J Crohns Colitis. 2023;17(Suppl_1):i764–5.
Silverberg JI, Bissonnette R, Kircik L, et al. Efficacy and safety of etrasimod, a sphingosine 1-phosphate receptor modulator, in adults with moderate-to-severe atopic dermatitis (ADVISE). J Eur Acad Dermatol Venereol. 2023;37(7):1366–74.
Guttman-Yassky E, Bissonette R, Kircik L, et al. Long-term efficacy and safety of etrasimod, an oral, selective sphingosine 1-phosphate receptor1,4,5 modulator, for moderate-to-severe atopic dermatitis: results from the open-label extension of the phase II ADVISE study [abstract no. 245 plus presentation]. Br J Dermatol. 2022;187(3):E128–9.
Vermeire S, Peyrin-Biroulet L, Panés J, et al. Etrasimod for the treatment of ulcerative colitis: up to 25 years of pooled safety data from global clinical trials [abstract no. P490 plus presentation]. J Crohns Colitis. 2023;17(Suppl_1):i619–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Matt Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirley, M. Etrasimod: First Approval. Drugs 84, 247–254 (2024). https://doi.org/10.1007/s40265-024-01997-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-024-01997-7