Abstract
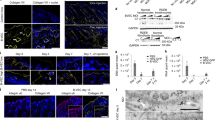
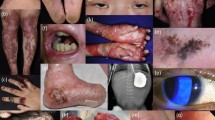
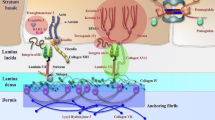
Beremagene geperpavec-svdt (VYJUVEK™) is a topically applied, redosable, live, replication defective herpes simplex virus-1 (HSV-1) vector -based gene therapy that is being developed by Krystal Biotech to deliver functional human collagen type VII alpha 1 chain (COL7A1) genes in patients with both, dominant and recessive dystrophic epidermolysis bullosa. Beremagene geperpavec can transduce both keratinocytes and fibroblasts and restore functional COL7 protein. In May 2023, beremagene geperpavec received its first approval in the US for the treatment of wounds in patients ≥ 6 months of age with dystrophic epidermolysis bullosa with mutation(s) in the COL7A1 gene. A Marketing Authorization Application for beremagene geperpavec in Europe is planned for the second half of 2023. This article summarizes the milestones in the development of beremagene geperpavec leading to this first approval for dystrophic epidermolysis bullosa.
Similar content being viewed by others
References
Schaffer DVPD. The coming of age of topical gene therapy for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387(24):2279–80.
Parry T, Krishnan S, Prosdocimo DA. A new era of in vivo gene therapy: the applicability of a differentiated HSV-1 based vector platform for redosable medicines. Cell Gene Ther Insights. 2022;8(5):641–51.
Feinstein JA, Bruckner AL, Chastek B, et al. Clinical characteristics, healthcare use, and annual costs among patients with dystrophic epidermolysis bullosa. Orphanet J Rare Dis. 2022;17(1):367.
Guide SV, Gonzalez ME, Bağcı IS, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387(24):2211–9.
Krystal Biotech Inc. VYJUVEK™ (beremagene geperpavec-svdt): US prescribing information. 2023. https://www.krystallabel.com/pdf/vyjuvek-us-pi.pdf. Accessed 2 Jun 2023.
US Food & Drug Administration. FDA approves first topical gene therapy for treatment of wounds in patients with dystrophic epidermolysis bullosa [media release]. 19 May 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-topical-gene-therapy-treatment-wounds-patients-dystrophic-epidermolysis-bullosa.
Gurevich I, Agarwal P, Zhang P, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022;28(4):780–8.
Carroll K, Zhang P, Duermeyer MJ, et al. Topical application of beremagene geperpavec, an engineered herpes simplex virus type I-based gene therapy vector expressing type VII collagen, is safe and efficacious in a murine corneal wound model. In: DEBRA International Conference. 2021.
Bagci I, Momin N, Agostini B, et al. Long term use of topical beremagene geperpavec (B-VEC) in two patients with dystrophic epidermolysis bullosa [abstract no. 905 plus poster]. J Investig Dermatol. 2023;143(5 Suppl):S155.
Sabater AL, Tovar A, Gomez J, et al. Topical beremagene geperpavec (B-VEC) for the treatment of recurrent cicatrizing conjunctivitis in a patient with dystrophic epidermolysis bullosa. In: Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Beremagene Geperpavec: First Approval. Drugs 83, 1131–1135 (2023). https://doi.org/10.1007/s40265-023-01921-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01921-5