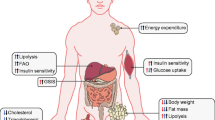
Abstract
Obesity, type 2 diabetes, and the numerous associated metabolic co-morbidities are growing global threats to public health. Despite recent progress in pharmacotherapies for metabolic diseases, the current treatment options have limited efficacy and provide mostly symptomatic relief with little or no impact on disease reversal. Thus, improved therapies are urgently needed. As a result, the scientific community has increasingly invested in leveraging new pathophysiological insights into more efficacious pharmacotherapies for metabolic complications. A heightened understanding of the large, interindividual variation in responsiveness to certain metabolic medicines combined with advances in engineering multi-agonist candidates are important steps towards this goal. Additionally, the emerging pharmacological concept of peptide-mediated targeting of small molecules for tissue-specific delivery holds promise for more powerful treatment solutions in the future. In this review, we summarize recent advances in medicinal chemistry and molecular pharmacology that have enabled the engineering of several, novel, poly-agonist drug candidates for treatment of metabolic diseases, and we discuss the recent results from clinical trials assessing the efficacy and safety of glucagon-like peptide (GLP)-1/glucagon and GLP-1/GIP co-agonists.




Similar content being viewed by others
References
World Health Organisation. Obesity and Overweight. (2018). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 28 Dec 2018.
Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;331:363–70.
González-Muniesa P, et al. Obesity. Nat Rev Dis Prim. 2017;3:1–6.
Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34.
Buchwald H, Aviador Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. Mayo Clin. 2018;292:1724–8.
Mingrone G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–73.
Adams TD, et al. After 6 years. JAMA. 2012;308:1122–31.
Buchwald H, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56.
Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. 2015;21:369–78.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601.
Chan JL, et al. Peptide YY levels are elevated after gastric bypass surgery. Obesity. 2006;14:194–8.
Chambers AP, et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144:50–2.
Myronovych A, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2014;22:390–400.
Kohli R, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J. Physiol Liver Physiol. 2010;299:G652–60.
Clemmensen C, et al. Emerging hormonal-based combination pharmacotherapies for the treatment of metabolic diseases. Nat Rev Endocrinol. 2019;15:90–104.
Clemmensen C, et al. Gut-brain cross-talk in metabolic control. Cell. 2017;168:758–74.
Pi-Sunyer X, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22.
O’Neil PM, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–49.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Le Roux CW, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–409.
Rosenstock J, et al. Effects of exenatide and lifestyle modification on bodyweight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173–5.
Blackman A, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the scale sleep apnea randomized clinical trial. Int J Obes. 2016;40:1310–9.
Davies MJ, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA J Am Med Assoc. 2015;314:687–99.
Wadden TA, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37:1443–51.
Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. 2018;14:390–403.
Marso SP, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Hernandez AF, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29.
Drucker DJ. The Cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30.
Kapitza C, et al. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive. Ethinylestradiol/Levonorgestrel J Clin Pharmacol. 2015;55:497–504.
Lau J, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58:7370–80.
Pratley RE, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275–86.
Holst JJ, Madsbad S. Semaglutide seems to be more effective the other GLP-1Ras. Ann Transl Med. 2017;5:505.
Campbell JE, Drucker DJ. Islet α cells and glucagon-critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11:329–38.
Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97:721–66.
Hjorth SA, Adelhorst K, Pedersen BB, Kirk O, Schwartz TW. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. J Biol Chem. 1994;269:30121–4.
Day JW, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749–57.
Pocai A, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258–66.
Tan TM, et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62:1131–8.
Cegla J, et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711–20.
Sánchez-Garrido MA, et al. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60:1851–61.
Ambery P, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607–18.
Henderson SJ, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab. 2016;18:1176–90.
Salem V, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18:72–81.
Finan B, et al. Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol Med. 2016;22:359–76.
Vilsbøll T, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide—regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–903.
Nauck MA, et al. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7.
Finan B, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:1–18.
Manandhar B, Ahn JM. Glucagon-like peptide-1 (GLP-1) analogs: recent advances, new possibilities, and therapeutic implications. J Med Chem. 2015;58:1020–37.
Frias JP, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343–52.
Frias JP, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–93.
Coskun T, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14.
Killion EA, et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med. 2018;10:1–11.
Mroz PA, et al. Optimized GIP analogs promote bodyweight lowering in mice through GIPR agonism not antagonism. Mol Metab. 2019;20:51–62.
Finan B, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27–36.
Jall S, et al. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol Metab. 2017;6:440–6.
Tschöp MH, et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 2016;24:51–62.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–80.
Dugger SA, Platt A, Goldstein DB. Drug development in the era of precision medicine. Nat Rev Drug Discov. 2018;17:183–96.
He R, Finan B, Mayer JP, Dimarchi RD. Peptide conjugates with small molecules designed to enhance efficacy and safety. Molecules. 2019;24:1855–88.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16:315–37.
Cancer Discovery. Lutetium Lu 177 dotatate approved by FDA. (2018). http://cancerdiscovery.aacrjournals.org/content/8/4/OF2.abstract. Accessed 3 June 2019.
Finan B, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–56.
Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11.
Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. 2011;22:24–33.
Gao Q, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94.
Martínez De Morentin PB, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53.
Zhou Z, et al. Estrogen receptor protects pancreatic -cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J Biol Chem. 2018;293:4735–51.
Vogel H, et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and bodyweight. Neuropharmacology. 2016;110:396–406.
Cao X, et al. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J Clin Invest. 2014;124:4351–62.
Tiano JP, Tate CR, Yang BS, Dimarchi R, Mauvais-Jarvis F. Effect of targeted estrogen delivery using glucagon-like peptide-1 on insulin secretion, insulin sensitivity and glucose homeostasis. Sci Rep. 2015;5:1–8.
Schwenk RW, et al. GLP-1–oestrogen attenuates hyperphagia and protects from beta cell failure in diabetes-prone New Zealand obese (NZO) mice. Diabetologia. 2014;58:604–14.
Drucker DJ. The ascending GLP-1 road from clinical safety to reduction of cardiovascular complications. Diabetes. 2018;67:1710–9.
Richards P, et al. Identification and characterization of GLP-1 receptor—expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–33.
Quarta C, et al. Molecular integration of incretin and glucocorticoid action reverses immunometabolic dysfunction and obesity. Cell Metab. 2017;26:620–32 (e6).
Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans Find the latest version: obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62.
Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab. 2017;6:366–73.
Gregor MF, Hotamisligil S. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45.
Décarie-Spain L, Fisette A, Zhu Z, Yang B, DiMarchi RD, Tschöp MH, Finan B, Fulton S, Clemmensen C. GLP-1/dexamethasone inhibits food reward without inducing mood and memory deficits in mice. Neuropharmacology. 2019;151:55–63.
Finan B, et al. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell. 2016;167:843–57 (e14).
Martínez-Sánchez N, et al. Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017;26:212–29 (e12).
Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259–69.
Jabbar A, et al. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. 2016;14:39–55.
Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51.
Hung G, et al. Characterization of target mRNA reduction through in situ RNA hybridization in multiple organ systems following systemic antisense treatment in animals. Nucleic Acid Ther. 2013;23:369–78.
Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14:9–22.
Ämmälä C, et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci Adv. 2018;4:eaat3386.
Andreassen KV, et al. A novel oral dual amylin and calcitonin receptor agonist (KBP-042) exerts antiobesity and antidiabetic effects in rats. Am J Physiol Metab. 2014;307:E24–33.
Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required. Br J Cancer. 2017;117:1736–42.
Erickson HK, et al. Tumor delivery and in vivo processing of disulfide-linked and thioether-linked antibody—maytansinoid conjugates. Bioconjugate Chem Chem. 2010;21:84–92.
Doronina SO, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotech. 2003;21:778–84.
Kellogg BA, et al. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug Chem. 2011;22:717–27.
Dubowchik GM, et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002;13:855–69.
Jeffrey SC, et al. Development and properties of β-glucuronide linkers for monoclonal antibody-drug conjugates. Bioconjug Chem. 2006;17:831–40.
Kern JC, et al. Discovery of pyrophosphate diesters as tunable, soluble, and bioorthogonal linkers for site-specific antibody-drug conjugates. J Am Chem Soc. 2016;138:1430–45.
Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med. 2017;58:61–6.
Wild D, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55:1248–53.
Chodorge M, et al. Engineering of a GLP-1 analogue peptide/anti-PCSK9 antibody fusion for type 2 diabetes treatment. Sci Rep. 2018;8:17545.
Jain M, et al. Randomised, phase 1, dose-finding study of MEDI4166, a PCSK9 antibody and GLP-1 analogue fusion molecule, in overweight or obese patients with type 2 diabetes mellitus. Diabetologia. 2019;62:373–86.
Herrath M Von, et al. Case reports of pre-clinical replication studies in metabolism and diabetes. Cell Metab. 2019;29:795–802.
Kleinert M, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14:140–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
CC receives funding from the Lundbeck Foundation (Fellowship: R238-2016-2859) and the Novo Nordisk Foundation (NNF17OC0026114). KS receives funding from the Lundbeck Foundation. Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark and partially funded by an unconditional donation from the Novo Nordisk Foundation.
Conflicts of interest
Jonas Petersen, Bente Frølund, Kristian Strømgaard and Christoffer Clemmensen declare no conflicts of interest that are directly relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Petersen, J., Strømgaard, K., Frølund, B. et al. Designing Poly-agonists for Treatment of Metabolic Diseases: Challenges and Opportunities. Drugs 79, 1187–1197 (2019). https://doi.org/10.1007/s40265-019-01153-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01153-6