Abstract
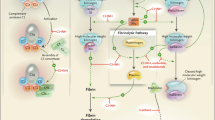
Shire is developing lanadelumab (Takhzyro™) for the prevention of hereditary angioedema (HAE) attacks. Lanadelumab is a fully human monoclonal antibody that inhibits plasma kallikrein. Mutations in the SERPING1 gene lead to C1 inhibitor deficiency or dysfunction, resulting in uncontrolled plasma kallikrein activity, which in turn produces excessive bradykinin, a vasodilator thought to cause angioedema symptoms. Subcutaneous administration of lanadelumab significantly reduced HAE attacks versus placebo in patients aged ≥ 12 years with type I or II HAE in a phase III trial. Based on these results, lanadelumab is recently approved in the USA for the prevention of HAE attacks in patients aged ≥ 12 years. It is also preregistered in the EU, Canada, Australia and Switzerland. This article summarizes the milestones in the development of lanadelumab leading to this first approval.
Similar content being viewed by others
References
Takhzyro™ (lanadelumab-flyo) injection, for subcutaneous use: US prescribing information; 2018. https://www.fda.gov. Accessed 5 Sep 2018.
Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-the 2017 revision and update. Allergy. 2018;73(8):1575–96.
Banerji A, Busse P, Shennak M, et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. N Engl J Med. 2017;376(8):717–28.
Dyax. Dyax announces positive results from phase 1b clinical trial of DX-2930 [media release]. 31 Mar 2015. http://www.dyax.com.
Shire. FDA accepts Shire’s BLA and grants priority review for lanadelumab for the prevention of attacks in HAE patients [media release]. 23 Feb 2018. http://www.shire.com.
Shire. The EMA grants accelerated assessment for Shire’s lanadelumab being evaluated for the prevention of attacks in HAE patients aged 12 years and older [media release]. 27 Feb 2018. http://www.shire.com.
European Medicines Agency. Public summary of opinion on orphan designation: recombinant human IgG1 kappa light chain monoclonal antibody targeting plasma kallikrein for the treatment of hereditary angioedema; 2015. http://www.ema.europa.eu. Accessed 5 Sep 2018.
Dyax. Dyax receives orphan drug designation from the U.S. FDA for DX-2930 in hereditary angioedema [media release]. 5 Dec 2013. http://www.dyax.com.
Shire. The Swiss agency for therapeutic products (Swissmedic) validates Shire’s marketing authorization application (MAA) for investigational hereditary angioedema (HAE) treatment tanadelumab [media release]. 18 Apr 2018. http://www.shire.com.
Dyax. Dyax Corp. receives FDA breakthrough therapy designation for DX-2930 for prevention of attacks of hereditary angioedema [media release]. 7 Jul 2015. http://www.dyax.com.
Shire. Shire completes acquisition of Dyax [media release]. 22 Jan 2016. http://www.shire.com.
Dyax. Dyax Corp. provides update on DX-2930 clinical and commercial supply initiatives [media release]. 12 Aug 2015. http://www.dyax.com.
Dyax. Dyax Corp. announces issuance of two key U.S. patents for DX-2930 [media release]. 15 sep 2014. http://www.dyax.com.
Sexton DJ, Faucette R, Kenniston J, et al. Approaches to estimate plasma kallikrein inhibition levels required for attack prophylaxis in hereditary angioedema [abstract no. 1055]. Allergy. 2017;72(Suppl 103):596.
Chyung Y, Vince B, Iarrobino R, et al. A phase 1 study investigating DX-2930 in healthy subjects. Ann Allergy Asthma Immunol. 2014;113(4):460–466.e2.
Lumry W, Cicardi M, Busse P, et al. Attack frequency, C1-INH function, and levels of cleaved kininogen do not influence the clinical response to DX-2930 in patients with hereditary angioedema [abstract no. 50]. Ann Allergy Asthma Immunol. 2015;115(5 Suppl):A31.
Chyung Y, Sexton D, TenHoor C, et al. Pharmacologic modeling to guide the DX-2930 dosing regimen in investigating long-term prophylaxis of hereditary angioedema [abstract no. P289]. Ann Allergy Asthma Immunol. 2014;113(5 Suppl):A106.
Wedner HJ, Busse PJ, Banerji A, et al. Modeling and analyses to identify potential dosing regimens of DX-2930 for the long-term prophylaxis of hereditary angioedema [abstract no. 822]. J Allergy Clin Immunol. 2016;137(2 Suppl):AB252.
Riedl MA, Tachdjian R, Schranz J, et al. Consistent lanadelumab treatment effect in patients with hereditary angioedema (HAE) regardless of baseline attack frequency in the phase 3 HELP study [abstract no. 151]. J Allergy Clin Immunol. 2018;141(2 Suppl):AB47.
Johnston DT, Anderson JT, Schranz J, et al. Efficacy of lanadelumab in patients switching from long-term prophylaxis with C1-inhibitor (C1-INH): results from the phase 3 HELP study [abstract no. 150]. J Allergy Clin Immunol. 2018;141(2 Suppl):AB47.
Riedl MA, Bernstein JA, Craig T, et al. An open-label study to evaluate the long-term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension. Clin Transl Allergy. 2017. https://doi.org/10.1186/s13601-017-0172-9.
Lumry WR, Weller K, Magerl M, et al. Lanadelumab markedly improves health-related quality of life in hereditary angioedema patients in the HELP study [abstract no. 152]. J Allergy Clin Immunol. 2018;141(2 Suppl):AB47.
Shire. Shire announces FDA approval of TAKHZYRO™ (lanadelumab-flyo), a first-of-its-kind mAb preventive treatment for hereditary angioedema [media release]. 23 Aug 2018. https://www.shire.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Lanadelumab: First Global Approval. Drugs 78, 1633–1637 (2018). https://doi.org/10.1007/s40265-018-0987-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0987-2