Abstract
Background
Second-generation direct-acting antiviral agents are integral to treatment of hepatitis C (HCV) infection. Eight-week courses of ledipasvir/sofosbuvir (LDV/SOF) have been supported in some studies, but data are limited on efficacy in real-world use. Controversy exists regarding applicability of clinical trials to real-world effectiveness. We report virologic responses of patients with HCV genotype 1 infection receiving LDV/SOF for 8 or 12 weeks in a large integrated healthcare system.
Methods
All patients receiving LDV/SOF, without ribavirin, were identified from pharmacy records, and outcomes are reported. Only treatment-naïve patients without evidence of cirrhosis and hepatitis C viral load less than 6 million IU/ml were candidates for 8-week therapy. Treatment was at clinician discretion, but delivered by a multidisciplinary team and reviewed for appropriateness and adherence to these criteria by one of the authors, all experienced in hepatitis C treatment. Sustained viral response at 12 weeks (SVR 12) was contrasted between those receiving 8 and those receiving 12 weeks of treatment.
Results
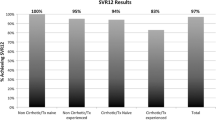
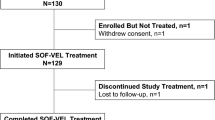
Completed prescriptions for LDV/SOF, without ribavirin, as of 30 September 2015 were identified in 1021 patients. Five patients discontinued therapy due to medical reasons and 35 had incomplete follow-up viral load data, thus there were 981 evaluable patients: 377 treated for 8 weeks and 604 treated for 12 weeks. SVR 12 was virtually identical at 93.6 and 93.5%, respectively. Baseline characteristics differed between the two groups, as only treatment-naïve, non-cirrhotic, non-HIV-infected patients were eligible for an 8-week course of therapy.
Conclusions
Eight-week courses of LDV/SOF are comparable to 12-week courses in real-world use among selected patients supported by a multidisciplinary team.
Similar content being viewed by others
References
Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib E, Ryan M, Rustgi V, Chojkier M, Herring R, DiBisceglie AM, Pockros PJ, Subramanian GM. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88. doi:10.1056/NEJMoa1402355.
Lawitz E, Poordad FF, Pang PD, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet. 2014;383:515–23. doi:10.1016/S0140-6736(13)62121-2.
Ingiliz P, Christensen S, Kimhofer T, Hueppe D, Lutz T, Schewe K, Busch H, Schmutz G, Wehmeyer MH, Boesecke C, Simon K, Berger F, Rockstroh JK, Schulze zur Wiesch J, Baumgarten A, Mauss S. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus infection in HCV-mono-infected and HIV-HCV co-infected individuals—results from the German hepatitis C cohort (GECCO-01). Clin Infect Dis. First published online August 17, 2016 doi:10.1093/cid/ciw567.
Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405–14. doi:10.1002/hep.28625.
Terrault NA, Zeuzem S, Di Bisceglie AM, Lim JK, Pockros PJ, Frazier LM, Kuo A, Lok AS, Shiffman ML, Ben Ari Z, Akushevich L, Vainorius M, Sulkowski MS, Fried MW, Nelson DR, for the HCV-TARGET study group. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016. doi:10.1053/j.gastro.2016.08.004.
Barua S, Greenwald R, Grebely R, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215–23. doi:10.7326/M15-0406.
Elting LE, Cooksley C, Bekele BN, Frumovitz M, Avritscher EBC, Sun C, Bodurka DC. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106(11):2452–8. doi:10.1002/cncr.21907.
Kaul S, Diamond GA, Weintraub WS. Trials and tribulations of non-inferiority: the ximelagatran experience. J Am Coll Cardiol. 2005;46:1986–95. doi:10.1016/j.jacc.2005.07.062.
Nallamothu BK, Hayward RA, Bates ER. Cardiovascular therapies: beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–303. doi:10.1161/CIRCULATIONAHA.107.703579.
Weiss NS, Koepsell TS, Psaty BM. Generalizability of the results of randomized trials. Arch Intern Med. 2008;168(2):133–5. doi:10.1001/archinternmed.2007.30.
Lai JB, Witt DJ, Witt MA. Real-world effectiveness of sofosbuvir (SOF), telaprevir and boceprevir (T, B) based therapy for hepatitis C virus (HCV): an analysis in a large integrated health care system. The International Liver Congress, April 23, 2015, Vienna, Austria (Abstract).
Truong E, Lai J, Witt D. Clinical management of HIV-positive patients by a HIV-specialist physician with or without clinical pharmacist support. Annual Seminar, California Society Health-System Pharmacists. Anaheim, CA October 9, 2008 (Abstract).
Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16(2):372–6. doi:10.1007/s11894-014-0372-6.
Cloherty G, Cohen D, Sarrazin C, Wedemeyer H, Chevaliez S, Herman C, Bernstein B, Pawlotsky JM. HCV RNA assay sensitivity impacts the management of patients treated with direct-acting antivirals. Antivir Ther. 2015;20(2):177–83. doi:10.3851/IMP2810.
http://micromedex.com/products/product-suites/clinical-knowledge/redbook. Accessed 27 Dec 2016.
Available at: http://www.bloomberg.com/news/articles/2015-01-12/prime-covers-both-gilead-and-abbvie-liver-drugs-as-prices-plunge. Accessed 6 March 2016.
Horberg MA, Hurley LB, Towner WJ, Allerton WJ, Tang BT, Catz SL, Silverberg MJ, Quesenberry CP. Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2012;60(2):183–90. doi:10.1097/QAI.0b013e31824bd605.
Horberg MA, Hurley LB, Towner WJ, Allerton MW, Tang BT, Catz SL, Silverberg MJ, Quesenberry CP. Influence of provider experience on antiretroviral adherence and viral suppression. HIV/AIDS Res Palliat Care. 2012;4:125–33. doi:10.2147/HIV.S35174.
Chidi AP, Rogal S, Bryce CL, Fine MJ, Good CB, Myaskovsky L, Rustgi VK, Smith KJ. Cost-effectiveness of new antiviral regimens for treatment-naïve U.S. veterans with hepatitis C. Hepatology. 2016;63:428–36. doi:10.1002/hep.28327.
AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed 25 July 2016.
https://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf.
O’Brien TR, Lang Kuhs KA, Pfeiffer RM. Subgroup differences in response to 8 weeks of ledipasvir/sofosbuvir for chronic hepatitis C. Open Forum Infect Dis. 2014;1(3):1–3. doi:10.1093/ofid/ofu110.
AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;2015(62):932–54. doi:10.1002/hep.27950.
Wilder JM, Jeffers LJ, Ravendhran N, Shiffman ML, Poulos J, Sulkowski MS, Gitlin N, et al. Safety and efficacy of ledipasvir-sofosbuvir in Black patients with hepatitis c virus infection: a retrospective analysis of phase 3 data. Hepatology. 2016;63(2):437–44. doi:10.1002/hep.28334.
http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed 17 Dec 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding/conflict of interest
None of the authors have any conflicts of interest. There was no funding for this project.
Rights and permissions
About this article
Cite this article
Lai, J.B., Witt, M.A., Pauly, M.P. et al. Eight- or 12-Week Treatment of Hepatitis C with Ledipasvir/Sofosbuvir: Real-World Experience in a Large Integrated Health System. Drugs 77, 313–318 (2017). https://doi.org/10.1007/s40265-016-0684-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0684-y