Abstract
Introduction and Objective
In May 2018, the World Health Organization and other regulatory authorities released a safety alert for dolutegravir related to a risk of neural tube defects among women exposed to dolutegravir at the time of conception. Models of how drug safety information can be shared effectively in the shortest time are necessary to prevent interruptions of public health programs. We sought to describe an implementation process to inform and support women already on dolutegravir-based regimens at the time of conception to make informed choices following the safety alert of a potential teratogenicity risk. We describe the choices made by women, as well as determine the factors associated with women’s choices to switch off dolutegravir.
Methods
A clinic response plan was developed in the first week following the alert and clinic staff were trained on safety guidance. All women aged < 55 years taking dolutegravir were identified from the clinic database and contacted by phone for earlier appointments. Non-menopausal and non-surgically sterilized women were referred for urine pregnancy testing and evaluation of pregnancy intentions in the following 12 months and effective family planning was offered. We describe the coverage of women who received the communication as well as the fidelity to the outlined plan from 21 May to 12 September, 2018. We used modified a Poisson regression analysis to determine factors associated with switching off dolutegravir.
Results
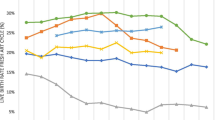
Of all active patients in the clinic, 9% (690/7963) were identified as female aged < 55 years taking dolutegravir. Ninety-five percent (656/690) were reviewed by September 2018 and informed of the safety alert, implying a high level of uptake. Fidelity to standard operating procedures was also high at 72%. Twenty-two percent (146/656) of patients were menopausal or surgically sterilized. Five hundred and ten women were of reproductive potential with a median age (interquartile range) of 37 years (30–42 years). Five percent (23/510) were human chorionic gonadotrophin positive and all initial ultrasound reports revealed no deformities. Twenty-one percent (108/510) had intentions to conceive and opted to stop taking dolutegravir with 90% (97/108) switching to efavirenz. Seventy-nine percent (402/510) opted to remain taking dolutegravir. However, only 40% (160/402) chose effective contraceptive methods and 60% (242/402) opted for condoms only/no contraceptive method.
Conclusions
A rapid well-coordinated response ensured prompt communication of the dolutegravir safety warning. The process developed by the clinic can act as a model for response during drug safety alerts. Women made informed decisions with most opting to remain taking dolutegravir; however, effective contraception uptake was low.





Similar content being viewed by others
References
UNAIDS. UNAIDS data 2018. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed 05 Jul 2020.
WHO. Guidelines on the public health response to pretreatment HIV drug resistance. July 2017. https://apps.who.int/iris/bitstream/handle/10665/255880/9789241550055-eng.pdf;jsessionid=C3EE93B78182C3FD322531B461143CBA?sequence=1. Accessed 01 Feb 2019.
Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med. 2018;379(10):979–81.
WHO. Statement on dolutegravir. https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf. Accessed 01 Feb 2019.
Dodoo A, Hugman B. Risk perception and communication in sub-Saharan Africa. Drug Saf. 2012;35(11):1041–52.
Campbell WH, Califf RM. Improving communication of drug risks to prevent patient injury: proceedings of a workshop. Pharmacoepidemiol Drug Saf. 2003;12(3):183–94.
Isah AO, Pal SN, Olsson S, et al. Specific features of medicines safety and pharmacovigilance in Africa. Ther Adv Drug Saf. 2012;3(1):25–34.
Nwaka S, Ochem A, Besson D, et al. Analysis of pan-African Centres of excellence in health innovation highlights opportunities and challenges for local innovation and financing in the continent. BMC Int Health Hum Rights. 2012;12(1):11.
Castelnuovo B, Kiragga A, Afayo V, et al. Implementation of provider-based electronic medical records and improvement of the quality of data in a large HIV program in sub-Saharan Africa. PLoS One. 2012;7(12):e51631.
The Antiretroviral Pregnancy Registry. http://www.apregistry.com/. Accessed 05 Jul 2020.
Sweden K, Kiiza D, Laker E, et al. High prevalence and long duration of nervous system and psychiatric adverse drug reactions in Ugandan patients taking efavirenz 600 mg daily. J Antimicrob Chemother. 2018;73(11):3158–61.
Edwards A, Elwyn G. Understanding risk and lessons for clinical risk communication about treatment preferences. Qual Health Care. 2001;10(Suppl. 1):i9–13.
AfroCab. IAS2018: communique of the Kigali dolutegravir stakeholder meeting of African women living with HIV, hosted by AfroCAB. 2018, Kigali, Rwanda 13–14 July 2020.
Trust S. Building a safe house on firm ground: key findings from a global values and preferences survey regarding the sexual and reproductive health and human rights of women living with HIV. Geneva: WHO; 2014.
Thomson KA, Dhanireddy S, Andrasik M, et al. Fertility desires and preferences for safer conception strategies among people receiving care for HIV at a publicly-funded clinic in Seattle, WA. AIDS Care. 2018;30(1):121–9.
Finocchario-Kessler S, Dariotis JK, Sweat MD, et al. Do HIV-infected women want to discuss reproductive plans with providers, and are those conversations occurring? AIDS Patient Care STDS. 2010;24(5):317–23.
Schwartz SR, Rees H, Mehta S, et al. High incidence of unplanned pregnancy after antiretroviral therapy initiation: findings from a prospective cohort study in South Africa. PLoS One. 2012;7(4):e36039.
Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381(9):827–40.
WHO. Update of recommendations on first- and second-line antiretroviral regimes. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1. Accessed 24 Sept 2019.
Acknowledgements
We acknowledge the clinic staff who contributed to the effective roll out of this project including Farida Mayanja, Geraldine Kisa, Elizabeth Tindyebwa, Joseph Mawejje, Swalha Namanda, Eriphase Mugabi, and Leah Mbabazi. We also acknowledge the patients we provided care for at the Infectious Diseases Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding obtained for this study.
Conflict of interest
Mohammed Lamorde reports grants and non-financial support from ViiV, grants and personal fees from Mylan, and grants from Janssen Pharmaceuticals, outside the submitted work. Eva Agnes Odongpiny Laker, Arnold Arinaitwe, Noela Owarwo, Annet Onzia, Benson Nasasira, Abdullah Wailagala, Ivan Kalule, Godwin Anguzu, Agnes Kiragga, Kay Seden, Isaac Lwanga, Barbara Castelnuovo, and Rachel Musomba have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Analysis and publication of data obtained from the Infectious Diseases Institute Adult Clinic are approved and annually renewed by the School of Medicine Research and Ethics Committee, Makerere University Medical School (reference no. 2009-120) and the Uganda National Council for Science and Technology. All information is analyzed after removing unique personal identifiers.
Consent to Participate
Patients provided written or oral consent to participate in the study.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
EL had final responsibility for the decision to publish. AA, NO, OA, NB, WA, AG, KS, RM, AG, IL, RM, BC, and ML all contributed to the design, data analysis, and interpretation of results. AG and AK were responsible for data extraction from the clinic database. EL, AA, NO, OA, NB, WA, KS, RM, AG, IL, RM, BC, and ML contributed to the drafting of the manuscript, reviewed the paper for substantial intellectual content, and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laker, E.A.O., Arinaitwe, A., Owarwo, N. et al. The Potential Teratogenicity Alert for Women Conceiving on Dolutegravir-Based Regimens: An Assessment of Risk Communication by an Urban HIV Clinic in Uganda and Choices made by Women. Drug Saf 43, 1133–1140 (2020). https://doi.org/10.1007/s40264-020-00974-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-020-00974-9